Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices
- PMID: 27226132
- PMCID: PMC6563337
- DOI: 10.1016/S0140-6736(16)30448-2
Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices
Abstract
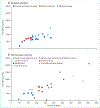
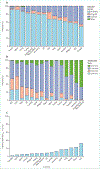
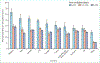
More than 2 million people worldwide are being treated for end-stage kidney disease (ESKD). This Series paper provides an overview of incidence, modality use (in-centre haemodialysis, home dialysis, or transplantation), and mortality for patients with ESKD based on national registry data. We also present data from an international cohort study to highlight differences in haemodialysis practices that affect survival and the experience of patients who rely on this therapy, which is both life-sustaining and profoundly disruptive to their quality of life. Data illustrate disparities in access to renal replacement therapy of any kind and in the use of transplantation or home dialysis, both of which are widely considered preferable to in-centre haemodialysis for many patients with ESKD in settings where infrastructure permits. For most patients with ESKD worldwide who are treated with in-centre haemodialysis, overall survival is poor, but longer in some Asian countries than elsewhere in the world, and longer in Europe than in the USA, although this gap has reduced. Commendable haemodialysis practice includes exceptionally high use of surgical vascular access in Japan and in some European countries, and the use of longer or more frequent dialysis sessions in some countries, allowing for more effective volume management. Mortality is especially high soon after ESKD onset, and improved preparation for ESKD is needed including alignment of decision making with the wishes of patients and families.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
BMR and RLP report grants from Amgen, Kyowa Hakko Kirin, Baxter Healthcare, European Renal Association/European Dialysis and Transplant Association (ERA-EDTA), Vifor Pharma Ltd, Hexal AG, AbbVie, Shire, German Society of Nephrology, Società Italiana di Nefrologia, Japanese Society for Peritoneal Dialysis, Keryx, and Genzyme Corporation. BMR reports personal fees from University of Toronto, Rhode Island Hospital, and Kyowa Hakko Kirin, outside of the submitted work. TA reports personal fees from Kyowa Hakko Kirin, and AbbVie Inc, during the conduct of the study; and reports personal fees from JT Pharmaceuticals Corporate, Kissei Pharmaceutical, Nipro Medical, Ono Pharmaceutical, Astellas, Bayer HealthCare, Chugai Pharmaceutical, Torii Pharmaceutical, Fuso Pharmaceutical, Teijin Pharma, and GlaxoSmithKline, outside of the submitted work. KJJ reports grants from ERA-EDTA, European Society for Paediatric Nephrology, International Pediatric Nephrology Association, and the Dutch Kidney Foundation, outside of the submitted work. PGK reports personal fees from Fresenius Australia and Quanta Fluid Solutions, outside of the submitted work. RLP reports personal fees from Kyowa Hakko Kirin and reports non-financial support from National Kidney Foundation, outside of the submitted work. RS declares no competing interests.
Figures


Similar articles
-
Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy.Am J Nephrol. 2021;52(2):98-107. doi: 10.1159/000514550. Epub 2021 Mar 22. Am J Nephrol. 2021. PMID: 33752206 Free PMC article. Review.
-
Vascular access in children requiring maintenance haemodialysis: a consensus document by the European Society for Paediatric Nephrology Dialysis Working Group.Nephrol Dial Transplant. 2019 Oct 1;34(10):1746-1765. doi: 10.1093/ndt/gfz011. Nephrol Dial Transplant. 2019. PMID: 30859187
-
Results of the European EDITH nephrologist survey on factors influencing treatment modality choice for end-stage kidney disease.Nephrol Dial Transplant. 2021 Dec 31;37(1):126-138. doi: 10.1093/ndt/gfaa342. Nephrol Dial Transplant. 2021. PMID: 33486525 Free PMC article.
-
A qualitative study on the lived experiences of individuals with end-stage kidney disease (ESKD) accessing haemodialysis in Northern Ghana.BMC Nephrol. 2024 May 31;25(1):186. doi: 10.1186/s12882-024-03622-x. BMC Nephrol. 2024. PMID: 38822254 Free PMC article.
-
Advance care planning for haemodialysis patients.Cochrane Database Syst Rev. 2016 Jul 26;7(7):CD010737. doi: 10.1002/14651858.CD010737.pub2. Cochrane Database Syst Rev. 2016. PMID: 27457661 Free PMC article. Review.
Cited by
-
Blood purification therapy in chronic renal failure and its impact on renal index, serological index, and inflammatory factors.Ann Med Surg (Lond). 2024 May 15;86(7):3856-3864. doi: 10.1097/MS9.0000000000002182. eCollection 2024 Jul. Ann Med Surg (Lond). 2024. PMID: 38989222 Free PMC article.
-
Efficacy of Balloon Angioplasty in Patients with Central Venous Stenosis or Obstruction Resulting from Central Vein Catheter Placement.Adv Biomed Res. 2023 Aug 31;12:223. doi: 10.4103/abr.abr_42_21. eCollection 2023. Adv Biomed Res. 2023. PMID: 38073745 Free PMC article.
-
Investigation of the effect of Jacobson's relaxation technique on the fatigue of family caregivers of hemodialysis patients: a single-blinded randomized controlled trial.Eur J Med Res. 2024 Jan 11;29(1):46. doi: 10.1186/s40001-024-01641-w. Eur J Med Res. 2024. PMID: 38212813 Free PMC article. Clinical Trial.
-
Fibroblast growth factor-23 correlates with advanced disease conditions and predicts high risk of major adverse cardiac and cerebral events in end-stage renal disease patients undergoing continuous ambulatory peritoneal dialysis.J Nephrol. 2019 Apr;32(2):307-314. doi: 10.1007/s40620-018-0557-4. Epub 2018 Nov 21. J Nephrol. 2019. PMID: 30465136
-
Changing landscape of dialysis withdrawal in patients with kidney failure: Implications for clinical practice.Nephrology (Carlton). 2022 Jul;27(7):551-565. doi: 10.1111/nep.14032. Epub 2022 Mar 6. Nephrology (Carlton). 2022. PMID: 35201646 Free PMC article. Review.
References
-
- Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–82. - PubMed
-
- US Renal Data System. 2015 USRDS Annual Data Report: epidemiology of kidney disease in the United States, vol 2 Bethesda, MD: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2015.
-
- Caskey FJ, Kramer A, Elliott RF, et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011; 26: 2604–10. - PubMed
-
- van de Luijtgaarden MW, Noordzij M, van Biesen W, et al. Conservative care in Europe--nephrologists’ experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant 2013; 28: 2604–12. - PubMed
-
- UK NHS. End of life care in adavanced kidney disease: a framework for Implementation http://www.ncpc.org.uk/sites/default/files/EndOfLifeCareInAdvancedKidney... (accessed March 7, 2016).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

