Prions: Beyond a Single Protein
- PMID: 27226089
- PMCID: PMC4978610
- DOI: 10.1128/CMR.00046-15
Prions: Beyond a Single Protein
Abstract
Since the term protein was first coined in 1838 and protein was discovered to be the essential component of fibrin and albumin, all cellular proteins were presumed to play beneficial roles in plants and mammals. However, in 1967, Griffith proposed that proteins could be infectious pathogens and postulated their involvement in scrapie, a universally fatal transmissible spongiform encephalopathy in goats and sheep. Nevertheless, this novel hypothesis had not been evidenced until 1982, when Prusiner and coworkers purified infectious particles from scrapie-infected hamster brains and demonstrated that they consisted of a specific protein that he called a "prion." Unprecedentedly, the infectious prion pathogen is actually derived from its endogenous cellular form in the central nervous system. Unlike other infectious agents, such as bacteria, viruses, and fungi, prions do not contain genetic materials such as DNA or RNA. The unique traits and genetic information of prions are believed to be encoded within the conformational structure and posttranslational modifications of the proteins. Remarkably, prion-like behavior has been recently observed in other cellular proteins-not only in pathogenic roles but also serving physiological functions. The significance of these fascinating developments in prion biology is far beyond the scope of a single cellular protein and its related disease.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
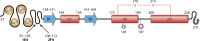
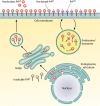
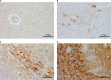
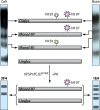


Similar articles
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
[Biology of non-conventional transmissible agents or prions].Rev Neurol (Paris). 1998 Feb;154(2):142-51. Rev Neurol (Paris). 1998. PMID: 9773035 Review. French.
-
Immunoaffinity purification and neutralization of scrapie prions.Prog Clin Biol Res. 1989;317:583-600. Prog Clin Biol Res. 1989. PMID: 2574871 Review.
-
Prion diseases of the central nervous system.Monogr Pathol. 1990;(32):86-122. Monogr Pathol. 1990. PMID: 2192281 Review.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
Cited by
-
Modulation of Neuroinflammation by the Gut Microbiota in Prion and Prion-Like Diseases.Pathogens. 2021 Jul 13;10(7):887. doi: 10.3390/pathogens10070887. Pathogens. 2021. PMID: 34358037 Free PMC article. Review.
-
Complex Networks of Prion-Like Proteins Reveal Cross Talk Between Stress and Memory Pathways in Plants.Front Plant Sci. 2021 Jul 26;12:707286. doi: 10.3389/fpls.2021.707286. eCollection 2021. Front Plant Sci. 2021. PMID: 34381483 Free PMC article.
-
Current and future applications of induced pluripotent stem cell-based models to study pathological proteins in neurodegenerative disorders.Mol Psychiatry. 2021 Jul;26(7):2685-2706. doi: 10.1038/s41380-020-00999-7. Epub 2021 Jan 25. Mol Psychiatry. 2021. PMID: 33495544 Free PMC article. Review.
-
Early preclinical detection of prions in the skin of prion-infected animals.Nat Commun. 2019 Jan 16;10(1):247. doi: 10.1038/s41467-018-08130-9. Nat Commun. 2019. PMID: 30651538 Free PMC article.
-
Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease.Sci Transl Med. 2017 Nov 22;9(417):eaam7785. doi: 10.1126/scitranslmed.aam7785. Sci Transl Med. 2017. PMID: 29167394 Free PMC article.
References
-
- Wickner RB. 2005. Scrapie in ancient China? Science 309:874. - PubMed
-
- Cuille J, Chelle PL. 1936. Pathologie animale—la maladie dite tremblante du mouton est-elle inoculable? CR Hebd Seances Acad Sci 203:1552–1554.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

