Skeletal myofiber VEGF regulates contraction-induced perfusion and exercise capacity but not muscle capillarity in adult mice
- PMID: 27225953
- PMCID: PMC4967234
- DOI: 10.1152/ajpregu.00533.2015
Skeletal myofiber VEGF regulates contraction-induced perfusion and exercise capacity but not muscle capillarity in adult mice
Abstract
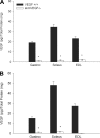
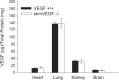
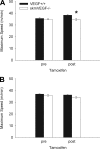
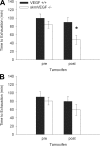
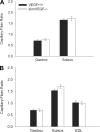
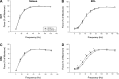
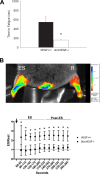
A single bout of exhaustive exercise signals expression of vascular endothelial growth factor (VEGF) in the exercising muscle. Previous studies have reported that mice with life-long deletion of skeletal myofiber VEGF have fewer capillaries and a severe reduction in endurance exercise. However, in adult mice, VEGF gene deletion conditionally targeted to skeletal myofibers limits exercise capacity without evidence of capillary regression. To explain this, we hypothesized that adult skeletal myofiber VEGF acutely regulates skeletal muscle perfusion during muscle contraction. A tamoxifen-inducible skeletal myofiber-specific VEGF gene deletion mouse (skmVEGF-/-) was used to reduce skeletal muscle VEGF protein by 90% in adult mice. Three weeks after inducing deletion of the skeletal myofiber VEGF gene, skmVEGF-/- mice exhibited diminished maximum running speed (-10%, P < 0.05) and endurance capacity (-47%; P < 0.05), which did not persist after 8 wk. In skmVEGF-/- mice, gastrocnemius complex time to fatigue measured in situ was 71% lower than control mice. Contraction-induced perfusion measured by optical imaging during a period of electrically stimulated muscle contraction was 85% lower in skmVEGF-/- than control mice. No evidence of capillary rarefication was detected in the soleus, gastrocnemius, and extensor digitorum longus (EDL) up to 8 wk after tamoxifen-induced VEGF ablation, and contractility and fatigue resistance of the soleus measured ex vivo were also unchanged. The force-frequency of the EDL showed a small right shift, but fatigue resistance did not differ between EDL from control and skmVEGF-/- mice. These data suggest myofiber VEGF is required for regulating perfusion during periods of contraction and may in this manner affect endurance capacity.
Keywords: exercise; fatigue; perfusion; skeletal myofiber; vascular endothelial growth factor.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Selective Life-Long Skeletal Myofiber-Targeted VEGF Gene Ablation Impairs Exercise Capacity in Adult Mice.J Cell Physiol. 2016 Feb;231(2):505-11. doi: 10.1002/jcp.25097. J Cell Physiol. 2016. PMID: 26201683
-
Skeletal myofiber VEGF is essential for the exercise training response in adult mice.Am J Physiol Regul Integr Comp Physiol. 2014 Apr 15;306(8):R586-95. doi: 10.1152/ajpregu.00522.2013. Epub 2014 Feb 12. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24523345 Free PMC article.
-
Skeletal myofiber VEGF deficiency leads to mitochondrial, structural, and contractile alterations in mouse diaphragm.J Appl Physiol (1985). 2019 Nov 1;127(5):1360-1369. doi: 10.1152/japplphysiol.00779.2018. Epub 2019 Sep 5. J Appl Physiol (1985). 2019. PMID: 31487223 Free PMC article.
-
The critical role of VEGF in skeletal muscle angiogenesis and blood flow.Biochem Soc Trans. 2011 Dec;39(6):1556-9. doi: 10.1042/BST20110646. Biochem Soc Trans. 2011. PMID: 22103486 Review.
-
Coupling of muscle metabolism and muscle blood flow in capillary units during contraction.Acta Physiol Scand. 2000 Apr;168(4):531-41. doi: 10.1046/j.1365-201x.2000.00706.x. Acta Physiol Scand. 2000. PMID: 10759590 Review.
Cited by
-
Human-like Cmah inactivation in mice increases running endurance and decreases muscle fatigability: implications for human evolution.Proc Biol Sci. 2018 Sep 12;285(1886):20181656. doi: 10.1098/rspb.2018.1656. Proc Biol Sci. 2018. PMID: 30209232 Free PMC article.
-
Lung transcriptomics reveals the underlying mechanism by which aerobic training enhances pulmonary function in chronic obstructive pulmonary disease.BMC Pulm Med. 2024 Mar 26;24(1):154. doi: 10.1186/s12890-024-02967-1. BMC Pulm Med. 2024. PMID: 38532405 Free PMC article.
-
Cigarette smoke directly impairs skeletal muscle function through capillary regression and altered myofibre calcium kinetics in mice.J Physiol. 2018 Jul;596(14):2901-2916. doi: 10.1113/JP275888. Epub 2018 Jun 19. J Physiol. 2018. PMID: 29797443 Free PMC article.
-
Heterogenous circulating miRNA changes in ME/CFS converge on a unified cluster of target genes: A computational analysis.PLoS One. 2023 Dec 29;18(12):e0296060. doi: 10.1371/journal.pone.0296060. eCollection 2023. PLoS One. 2023. PMID: 38157384 Free PMC article.
-
The role of the microcirculation in muscle function and plasticity.J Muscle Res Cell Motil. 2019 Jun;40(2):127-140. doi: 10.1007/s10974-019-09520-2. Epub 2019 Jun 5. J Muscle Res Cell Motil. 2019. PMID: 31165949 Free PMC article. Review.
References
-
- Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res 99: 715–722, 2006. - PubMed
-
- Ashrafpour H, Huang N, Neligan PC, Forrest CR, Addison PD, Moses MA, Levine RH, Pang CY. Vasodilator effect and mechanism of action of vascular endothelial growth factor in skin vasculature. Am J Physiol Heart Circ Physiol 286: H946–H954, 2004. - PubMed
-
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 98: 1258–1263, 2005. - PubMed
-
- Bradley EA, Eringa EC, Stehouwer CD, Korstjens I, van Nieuw Amerongen GP, Musters R, Sipkema P, Clark MG, Rattigan S. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol 30: 1137–1142, 2010. - PubMed
-
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol 81: 355–361, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

