Combinations of Oseltamivir and T-705 Extend the Treatment Window for Highly Pathogenic Influenza A(H5N1) Virus Infection in Mice
- PMID: 27221530
- PMCID: PMC4879667
- DOI: 10.1038/srep26742
Combinations of Oseltamivir and T-705 Extend the Treatment Window for Highly Pathogenic Influenza A(H5N1) Virus Infection in Mice
Abstract
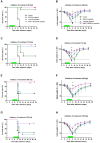
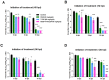
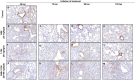
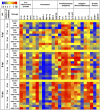
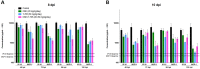
Current anti-influenza therapy depends on administering drugs soon after infection, which is often impractical. We assessed whether combinations of oseltamivir (a neuraminidase inhibitor) and T-705 (a nonspecific inhibitor of viral polymerases) could extend the window for treating lethal infection with highly pathogenic A(H5N1) influenza virus in mice. Combination therapy protected 100% of mice, even when delayed until 96 h postinoculation. Compared to animals receiving monotherapy, mice receiving combination therapy had reduced viral loads and restricted viral spread in lung tissues, limited lung damage, and decreased inflammatory cytokine production. Next-generation sequencing showed that virus populations in T-705-treated mice had greater genetic variability, with more frequent transversion events, than did populations in control and oseltamivir-treated mice, but no substitutions associated with resistance to oseltamivir or T-705 were detected. Thus, combination therapy extended the treatment window for A(H5N1) influenza infection in mice and should be considered for evaluation in a clinical setting.
Figures

Similar articles
-
Mycophenolic mofetil, an alternative antiviral and immunomodulator for the highly pathogenic avian influenza H5N1 virus infection.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):298-304. doi: 10.1016/j.bbrc.2017.10.037. Epub 2017 Oct 7. Biochem Biophys Res Commun. 2017. PMID: 29017920
-
Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice.Antivir Ther. 2007;12(3):363-70. Antivir Ther. 2007. PMID: 17591026
-
Combination Therapy with Oseltamivir and Favipiravir Delays Mortality but Does Not Prevent Oseltamivir Resistance in Immunodeficient Mice Infected with Pandemic A(H1N1) Influenza Virus.Viruses. 2018 Nov 3;10(11):610. doi: 10.3390/v10110610. Viruses. 2018. PMID: 30400276 Free PMC article.
-
Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice.Antimicrob Agents Chemother. 2008 Nov;52(11):3889-97. doi: 10.1128/AAC.01579-07. Epub 2008 Aug 25. Antimicrob Agents Chemother. 2008. PMID: 18725448 Free PMC article.
-
Neuraminidase inhibitor resistance in influenza viruses.J Med Virol. 2007 Oct;79(10):1577-86. doi: 10.1002/jmv.20951. J Med Virol. 2007. PMID: 17705169 Review.
Cited by
-
Favipiravir and Its Structural Analogs: Antiviral Activity and Synthesis Methods.Acta Naturae. 2022 Apr-Jun;14(2):16-38. doi: 10.32607/actanaturae.11652. Acta Naturae. 2022. PMID: 35923566 Free PMC article.
-
Influenza polymerase inhibitor resistance: Assessment of the current state of the art - A report of the isirv Antiviral group.Antiviral Res. 2021 Oct;194:105158. doi: 10.1016/j.antiviral.2021.105158. Epub 2021 Aug 4. Antiviral Res. 2021. PMID: 34363859 Free PMC article. Review.
-
A pharmacologically immunosuppressed mouse model for assessing influenza B virus pathogenicity and oseltamivir treatment.Antiviral Res. 2017 Dec;148:20-31. doi: 10.1016/j.antiviral.2017.10.021. Epub 2017 Oct 31. Antiviral Res. 2017. PMID: 29100887 Free PMC article.
-
Combination Therapy With Neuraminidase and Polymerase Inhibitors in Nude Mice Infected With Influenza Virus.J Infect Dis. 2018 Mar 5;217(6):887-896. doi: 10.1093/infdis/jix606. J Infect Dis. 2018. PMID: 29186472 Free PMC article.
-
Characterization of the In Vitro and In Vivo Efficacy of Baloxavir Marboxil against H5 Highly Pathogenic Avian Influenza Virus Infection.Viruses. 2022 Jan 8;14(1):111. doi: 10.3390/v14010111. Viruses. 2022. PMID: 35062315 Free PMC article.
References
-
- World Health Organization. Cumulative numbers of confirmed human cases for influenza A(H5N1) reported to WHO, 2003-2015 (2016). Available at: http://www.who.int/influenza/human_animal_interface/2016_01_20_tableH5N1.... (Accessed: 9th March 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

