Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin
- PMID: 27220888
- PMCID: PMC5129986
- DOI: 10.18632/oncotarget.9556
Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin
Erratum in
-
Correction: Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin.Oncotarget. 2024 Oct 1;15:697-698. doi: 10.18632/oncotarget.28657. Oncotarget. 2024. PMID: 39352802 Free PMC article. No abstract available.
Abstract
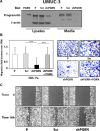
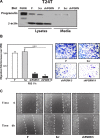
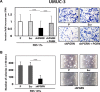
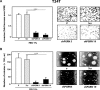
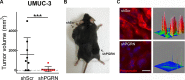
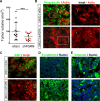
We have recently demonstrated a critical role for progranulin in bladder cancer. Progranulin contributes, as an autocrine growth factor, to the transformed phenotype by modulating Akt-and MAPK-driven motility, invasion and anchorage-independent growth. Progranulin also induces F-actin remodeling by interacting with the F-actin binding protein drebrin. In addition, progranulin is overexpressed in invasive bladder cancer compared to normal tissue controls, suggesting that progranulin might play a key role in driving the transition to the invasive phenotype of urothelial cancer. However, it is not established whether targeting progranulin could have therapeutic effects on bladder cancer. In this study, we stably depleted urothelial cancer cells of endogenous progranulin by shRNA approaches and determined that progranulin depletion severely inhibited the ability of tumorigenic urothelial cancer cells to migrate, invade and grow in anchorage-independency. We further demonstrate that progranulin expression is critical for tumor growth in vivo, in both xenograft and orthotopic tumor models. Notably, progranulin levels correlated with response to cisplatin treatment and were upregulated in bladder tumors. Our data indicate that progranulin may constitute a novel target for therapeutic intervention in bladder tumors. In addition, progranulin may serve as a novel biomarker for bladder cancer.
Keywords: anchorage-independent growth; bladder cancer; motility; progranulin; tumor formation in vivo.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

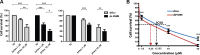

Similar articles
-
Progranulin/EphA2 axis: A novel oncogenic mechanism in bladder cancer.Matrix Biol. 2020 Nov;93:10-24. doi: 10.1016/j.matbio.2020.03.009. Epub 2020 May 15. Matrix Biol. 2020. PMID: 32417448 Free PMC article.
-
A novel role for drebrin in regulating progranulin bioactivity in bladder cancer.Oncotarget. 2015 May 10;6(13):10825-39. doi: 10.18632/oncotarget.3424. Oncotarget. 2015. PMID: 25839164 Free PMC article.
-
Correction: Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin.Oncotarget. 2024 Oct 1;15:697-698. doi: 10.18632/oncotarget.28657. Oncotarget. 2024. PMID: 39352802 Free PMC article. No abstract available.
-
Sortilin regulates progranulin action in castration-resistant prostate cancer cells.Endocrinology. 2015 Jan;156(1):58-70. doi: 10.1210/en.2014-1590. Endocrinology. 2015. PMID: 25365768 Free PMC article.
-
The perlecan-interacting growth factor progranulin regulates ubiquitination, sorting, and lysosomal degradation of sortilin.Matrix Biol. 2017 Dec;64:27-39. doi: 10.1016/j.matbio.2017.04.001. Epub 2017 Apr 20. Matrix Biol. 2017. PMID: 28433812 Free PMC article.
Cited by
-
Progranulin/EphA2 axis: A novel oncogenic mechanism in bladder cancer.Matrix Biol. 2020 Nov;93:10-24. doi: 10.1016/j.matbio.2020.03.009. Epub 2020 May 15. Matrix Biol. 2020. PMID: 32417448 Free PMC article.
-
Complexity of progranulin mechanisms of action in mesothelioma.J Exp Clin Cancer Res. 2022 Dec 5;41(1):333. doi: 10.1186/s13046-022-02546-4. J Exp Clin Cancer Res. 2022. PMID: 36471440 Free PMC article.
-
Prognostic significance of serum progranulin level in de novo adult acute lymphoblastic leukemia patients.Clin Exp Med. 2020 May;20(2):269-276. doi: 10.1007/s10238-020-00610-x. Epub 2020 Jan 31. Clin Exp Med. 2020. PMID: 32006270
-
Progranulin and its biological effects in cancer.Med Oncol. 2017 Nov 7;34(12):194. doi: 10.1007/s12032-017-1054-7. Med Oncol. 2017. PMID: 29116422 Free PMC article. Review.
-
Clinicopathological characteristics and outcomes of gastrointestinal stromal tumors with high progranulin expression.PLoS One. 2021 Jan 7;16(1):e0245153. doi: 10.1371/journal.pone.0245153. eCollection 2021. PLoS One. 2021. PMID: 33411849 Free PMC article. Clinical Trial.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Mitra AP, Cote RJ. Molecular Pathogenesis and Diagnostics of Bladder Cancer. Annu Rev Pathol. 2009;4:251–285. - PubMed
-
- Knowles MA. Molecular pathogenesis of bladder cancer. Int J Clin Oncol. 2008;13:287–297. - PubMed
-
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. - PubMed
-
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

