Promyelocytic leukemia protein isoform II inhibits infection by human adenovirus type 5 through effects on HSP70 and the interferon response
- PMID: 27217299
- PMCID: PMC5764125
- DOI: 10.1099/jgv.0.000510
Promyelocytic leukemia protein isoform II inhibits infection by human adenovirus type 5 through effects on HSP70 and the interferon response
Abstract
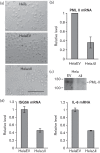
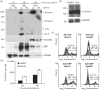
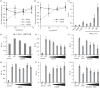
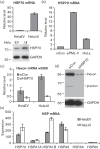
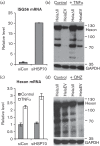
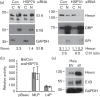
Promyelocytic leukemia (PML) proteins have been implicated in antiviral responses but PML and associated proteins are also suggested to support virus replication. One isoform, PML-II, is required for efficient transcription of interferon and interferon-responsive genes. We therefore investigated the PML-II contribution to human adenovirus 5 (Ad5) infection, using shRNA-mediated knockdown. HelaΔII cells showed a 2-3-fold elevation in Ad5 yield, reflecting an increase in late gene expression. This increase was found to be due in part to the reduced innate immune response consequent upon PML-II depletion. However, the effect was minor because the viral E4 Orf3 protein targets and inactivates this PML-II function. The major benefit to Ad5 in HelaΔII cells was exerted via an increase in HSP70; depletion of HSP70 completely reversed this replicative advantage. Increased Ad5 late gene expression was not due either to the previously described inhibition of inflammatory responses by HSP70 or to effects of HSP70 on major late promoter or L4 promoter activity, but might be linked to an observed increase in E1B 55K, as this protein is known to be required for efficient late gene expression. The induction of HSP70 by PML-II removal was specific for the HSPA1B gene among the HSP70 gene family and thus was not the consequence of a general stress response. Taken together, these data show that PML-II, through its various actions, has an overall negative effect on the Ad5 lifecycle.
Figures


Similar articles
-
Interaction of the adenovirus type 5 E4 Orf3 protein with promyelocytic leukemia protein isoform II is required for ND10 disruption.J Virol. 2006 Mar;80(6):3042-9. doi: 10.1128/JVI.80.6.3042-3049.2006. J Virol. 2006. PMID: 16501113 Free PMC article.
-
Incoming human papillomavirus 16 genome is lost in PML protein-deficient HaCaT keratinocytes.Cell Microbiol. 2017 May;19(5):10.1111/cmi.12708. doi: 10.1111/cmi.12708. Epub 2017 Jan 23. Cell Microbiol. 2017. PMID: 27860076 Free PMC article.
-
Promyelocytic Leukemia Protein Isoform II Promotes Transcription Factor Recruitment To Activate Interferon Beta and Interferon-Responsive Gene Expression.Mol Cell Biol. 2015 May;35(10):1660-72. doi: 10.1128/MCB.01478-14. Epub 2015 Mar 2. Mol Cell Biol. 2015. PMID: 25733689 Free PMC article.
-
Role of promyelocytic leukemia protein in host antiviral defense.J Interferon Cytokine Res. 2011 Jan;31(1):145-58. doi: 10.1089/jir.2010.0111. Epub 2011 Jan 3. J Interferon Cytokine Res. 2011. PMID: 21198351 Review.
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
Cited by
-
Crosstalk Between SUMO and Ubiquitin-Like Proteins: Implication for Antiviral Defense.Front Cell Dev Biol. 2021 Apr 21;9:671067. doi: 10.3389/fcell.2021.671067. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968942 Free PMC article. Review.
-
SARS-CoV-2 vaccine ChAdOx1 nCoV-19 infection of human cell lines reveals low levels of viral backbone gene transcription alongside very high levels of SARS-CoV-2 S glycoprotein gene transcription.Genome Med. 2021 Mar 15;13(1):43. doi: 10.1186/s13073-021-00859-1. Genome Med. 2021. PMID: 33722288 Free PMC article.
-
The Role of Nuclear Antiviral Factors against Invading DNA Viruses: The Immediate Fate of Incoming Viral Genomes.Viruses. 2016 Oct 22;8(10):290. doi: 10.3390/v8100290. Viruses. 2016. PMID: 27782081 Free PMC article. Review.
-
Low type I interferon response in COVID-19 patients: Interferon response may be a potential treatment for COVID-19.Biomed Rep. 2021 May;14(5):43. doi: 10.3892/br.2021.1419. Epub 2021 Mar 9. Biomed Rep. 2021. PMID: 33786172 Free PMC article.
-
Adenoviral strategies to overcome innate cellular responses to infection.FEBS Lett. 2019 Dec;593(24):3484-3495. doi: 10.1002/1873-3468.13680. Epub 2019 Nov 26. FEBS Lett. 2019. PMID: 31721176 Free PMC article. Review.
References
-
- Au W. C., Moore P. A., Lowther W., Juang Y. T., Pitha P. M.(1995). Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci U S A 9211657–11661. - PMC - PubMed
-
- Bernardi R., Pandolfi P. P.(2007). Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Revs Mol Cell Biol 81006–1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

