Mechanistic Approaches to Improve Correction of the Most Common Disease-Causing Mutation in Cystic Fibrosis
- PMID: 27214033
- PMCID: PMC4877091
- DOI: 10.1371/journal.pone.0155882
Mechanistic Approaches to Improve Correction of the Most Common Disease-Causing Mutation in Cystic Fibrosis
Abstract
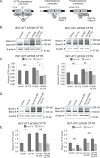
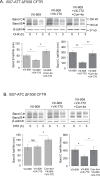
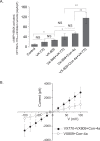
The most common mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene leads to deletion of the phenylalanine at position 508 (ΔF508) in the CFTR protein and causes multiple folding and functional defects. Contrary to large-scale efforts by industry and academia, no significant therapeutic benefit has been achieved with a single "corrector". Therefore, investigations concentrate on drug combinations. Orkambi (Vertex Pharmaceuticals), the first FDA-approved drug for treatment of cystic fibrosis (CF) caused by this mutation, is a combination of a corrector (VX-809) that facilitates ΔF508 CFTR biogenesis and a potentiator (VX-770), which improves its function. Yet, clinical trials utilizing this combination showed only modest therapeutic benefit. The low efficacy Orkambi has been attributed to VX-770-mediated destabilization of VX-809-rescued ΔF508 CFTR. Here we report that the negative effects of VX-770 can be reversed by increasing the half-life of the endoplasmic reticulum (ER) form (band B) of ΔF508 CFTR with another corrector (Corr-4a.) Although Corr-4a alone has only minimal effects on ΔF508 CFTR rescue, it increases the half-life of ΔF508 CFTR band B when it is present during half-life measurements. Our data shows that stabilization of band B ΔF508 CFTR with Corr-4a and simultaneous rescue with VX-809, leads to a >2-fold increase in cAMP-activated, CFTRinh-172-inhibited currents compared to VX-809 alone, or VX-809+VX-770. The negative effects of VX-770 and the Corr-4a protection are specific to the native I507-ATT ΔF508 CFTR without affecting the inherently more stable, synonymous variant I507-ATC ΔF508 CFTR. Our studies emphasize that stabilization of ΔF508 CFTR band B in the ER might improve its functional rescue by Orkambi.
Conflict of interest statement
Figures
Similar articles
-
A synonymous codon change alters the drug sensitivity of ΔF508 cystic fibrosis transmembrane conductance regulator.FASEB J. 2016 Jan;30(1):201-13. doi: 10.1096/fj.15-273714. Epub 2015 Sep 3. FASEB J. 2016. PMID: 26336913 Free PMC article.
-
Lipophilicity of the Cystic Fibrosis Drug, Ivacaftor (VX-770), and Its Destabilizing Effect on the Major CF-causing Mutation: F508del.Mol Pharmacol. 2018 Aug;94(2):917-925. doi: 10.1124/mol.118.112177. Epub 2018 Jun 14. Mol Pharmacol. 2018. PMID: 29903751
-
Restoration of R117H CFTR folding and function in human airway cells through combination treatment with VX-809 and VX-770.Am J Physiol Lung Cell Mol Physiol. 2016 Sep 1;311(3):L550-9. doi: 10.1152/ajplung.00186.2016. Epub 2016 Jul 8. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27402691 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment.Ann Pharmacother. 2012 Jul-Aug;46(7-8):1065-75. doi: 10.1345/aph.1R076. Epub 2012 Jun 26. Ann Pharmacother. 2012. PMID: 22739718 Review.
-
Lumacaftor/ivacaftor, a novel agent for the treatment of cystic fibrosis patients who are homozygous for the F580del CFTR mutation.Expert Rev Clin Pharmacol. 2017 Oct;10(10):1055-1072. doi: 10.1080/17512433.2017.1378094. Epub 2017 Sep 22. Expert Rev Clin Pharmacol. 2017. PMID: 28891346 Review.
Cited by
-
Improved fluorescence assays to measure the defects associated with F508del-CFTR allow identification of new active compounds.Br J Pharmacol. 2017 Apr;174(7):525-539. doi: 10.1111/bph.13715. Epub 2017 Feb 14. Br J Pharmacol. 2017. PMID: 28094839 Free PMC article.
-
[Interstitial processes of the lungs in childhood].Pathologe. 2017 Jul;38(4):260-271. doi: 10.1007/s00292-017-0280-2. Pathologe. 2017. PMID: 28349192 Review. German.
-
Net benefit of ivacaftor during prolonged tezacaftor/elexacaftor exposure in vitro.J Cyst Fibros. 2022 Jul;21(4):637-643. doi: 10.1016/j.jcf.2022.02.011. Epub 2022 Mar 2. J Cyst Fibros. 2022. PMID: 35248469 Free PMC article.
-
Oxidative and endoplasmic reticulum stress in respiratory disease.Clin Transl Immunology. 2018 Jun 13;7(6):e1019. doi: 10.1002/cti2.1019. eCollection 2018. Clin Transl Immunology. 2018. PMID: 29928501 Free PMC article. Review.
-
Matrine in association with FD‑2 stimulates F508del‑cystic fibrosis transmembrane conductance regulator activity in the presence of corrector VX809.Mol Med Rep. 2017 Dec;16(6):8849-8853. doi: 10.3892/mmr.2017.7736. Epub 2017 Oct 6. Mol Med Rep. 2017. PMID: 29039559 Free PMC article.
References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA [published erratum appears in Science 1989 Sep 29;245(4925):1437]. Science. 1989;245(4922):1066–73. - PubMed
-
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245(4922):1059–65. . - PubMed
-
- Zielenski J, Rozmahel R, Bozon D, Kerem B, Grzelczak Z, Riordan JR, et al. Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics. 1991;10(1):214–28. Epub 1991/05/01. . - PubMed
-
- Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994;269(41):25710–8. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

