Apoptosis in pancreatic β-islet cells in Type 2 diabetes
- PMID: 27209071
- PMCID: PMC4978108
- DOI: 10.17305/bjbms.2016.919
Apoptosis in pancreatic β-islet cells in Type 2 diabetes
Abstract
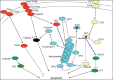
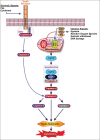
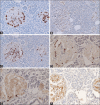
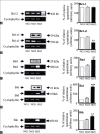
Apoptosis plays important roles in the pathophysiology of Type 2 diabetes mellitus (T2DM). The etiology of T2DM is multifactorial, including obesity-associated insulin resistance, defective insulin secretion, and loss of β-cell mass through β-cell apoptosis. β-cell apoptosis is mediated through a milliard of caspase family cascade machinery in T2DM. The glucose-induced insulin secretion is the principle pathophysiology of diabetes and insufficient insulin secretion results in chronic hyperglycemia, diabetes. Recently, hyperglycemia-induced β-cell apoptosis has been extensively studied on the balance of pro-apoptotic Bcl-2 proteins (Bad, Bid, Bik, and Bax) and anti-apoptotic Bcl family (Bcl-2 and Bcl-xL) toward apoptosis in vitro isolated islets and insulinoma cell culture. Apoptosis can only occur when the concentration of pro-apoptotic Bcl-2 exceeds that of anti-apoptotic proteins at the mitochondrial membrane of the intrinsic pathway. A bulk of recent research on hyperglycemia-induced apoptosis on β-cells unveiled complex details on glucose toxicity on β-cells in molecular levels coupled with cell membrane potential by adenosine triphosphate generation through K+ channel closure, opening Ca2+ channel and plasma membrane depolarization. Furthermore, animal models using knockout mice will shed light on the basic understanding of the pathophysiology of diabetes as a glucose metabolic disease complex, on the balance of anti-apoptotic Bcl family and pro-apoptotic genes. The cumulative knowledge will provide a better understanding of glucose metabolism at a molecular level and will lead to eventual prevention and therapeutic application for T2DM with improving medications.
Figures



Similar articles
-
The orphan nuclear receptor small heterodimer partner negatively regulates pancreatic beta cell survival and hyperglycemia in multiple low-dose streptozotocin-induced type 1 diabetic mice.Int J Biochem Cell Biol. 2013 Aug;45(8):1538-45. doi: 10.1016/j.biocel.2013.05.004. Epub 2013 May 13. Int J Biochem Cell Biol. 2013. PMID: 23680671
-
GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim.PLoS Genet. 2013 May;9(5):e1003532. doi: 10.1371/journal.pgen.1003532. Epub 2013 May 30. PLoS Genet. 2013. PMID: 23737756 Free PMC article.
-
Intracellular pathways of pancreatic β-cell apoptosis in type 1 diabetes.Diabetes Metab Res Rev. 2011 Nov;27(8):790-6. doi: 10.1002/dmrr.1253. Diabetes Metab Res Rev. 2011. PMID: 22069261
-
Protection of pancreatic beta-cells: is it feasible?Nutr Metab Cardiovasc Dis. 2008 Jan;18(1):74-83. doi: 10.1016/j.numecd.2007.05.004. Nutr Metab Cardiovasc Dis. 2008. PMID: 18096375 Review.
-
Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus.J Cell Biochem. 2013 Mar;114(3):525-31. doi: 10.1002/jcb.24402. J Cell Biochem. 2013. PMID: 22991242 Review.
Cited by
-
Improved meta-analysis pipeline ameliorates distinctive gene regulators of diabetic vasculopathy in human endothelial cell (hECs) RNA-Seq data.PLoS One. 2023 Nov 9;18(11):e0293939. doi: 10.1371/journal.pone.0293939. eCollection 2023. PLoS One. 2023. PMID: 37943808 Free PMC article.
-
TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis.Biosci Rep. 2016 Sep 29;36(5):e00386. doi: 10.1042/BSR20160135. Print 2016 Oct. Biosci Rep. 2016. PMID: 27515420 Free PMC article.
-
Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus.Evid Based Complement Alternat Med. 2021 Jul 14;2021:2920530. doi: 10.1155/2021/2920530. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34335803 Free PMC article. Review.
-
Anti-inflammatory effects of GLP-1 in patients with COVID-19.Expert Rev Anti Infect Ther. 2022 Mar;20(3):373-381. doi: 10.1080/14787210.2021.1964955. Epub 2021 Aug 12. Expert Rev Anti Infect Ther. 2022. PMID: 34348067 Free PMC article. Review.
-
Effect of pioglitazone on the expression of ubiquitin proteasome system and autophagic proteins in rat pancreas with metabolic syndrome.J Mol Histol. 2021 Oct;52(5):929-942. doi: 10.1007/s10735-021-10013-1. Epub 2021 Aug 19. J Mol Histol. 2021. PMID: 34410563
References
-
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–57. http://dx.doi.org/10.1038/bjc.1972.33 . - PMC - PubMed
-
- Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas: Estimation with a simple mathematical model. Diabetes. 1995;44(3):249–56. http://dx.doi.org/10.2337/diab.44.3.249 . - PubMed
-
- Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138(4):1736–41. http://dx.doi.org/10.1210/endo.138.4.5069 . - PubMed
-
- Mandrup-Poulsen T. beta-cell apoptosis: Stimuli and signaling. Diabetes. 2001;50(Suppl 1):S58–63. http://dx.doi.org/10.2337/diabetes.50.2007.S58 . - PubMed
-
- Lee SC, Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol. 2007;39(3):497–504. http://dx.doi.org/10.1016/j.biocel.2006.09.007 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

