In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection
- PMID: 27206407
- PMCID: PMC4875645
- DOI: 10.1186/s12977-016-0268-7
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection
Abstract
Background: The latent reservoir in resting CD4(+) T cells presents a major barrier to HIV cure. Latency-reversing agents are therefore being developed with the ultimate goal of disrupting the latent state, resulting in induction of HIV expression and clearance of infected cells. Histone deacetylase inhibitors (HDACi) have received a significant amount of attention for their potential as latency-reversing agents.
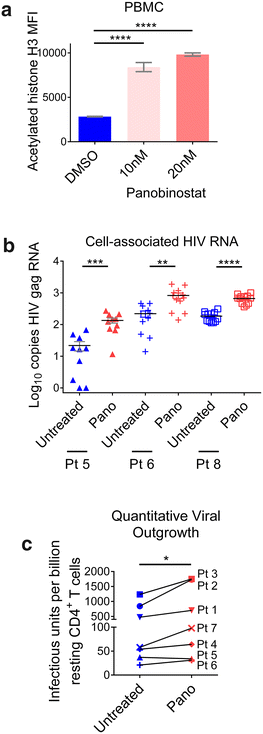
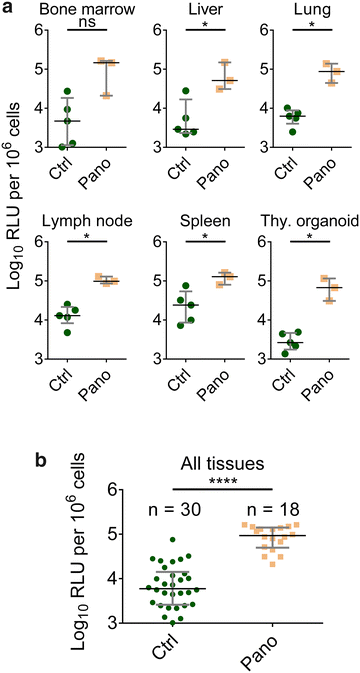
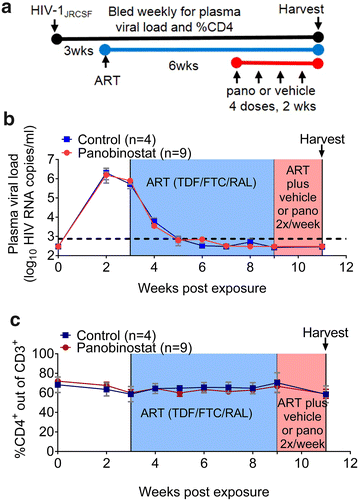
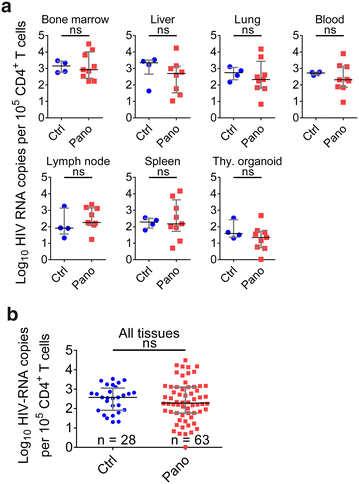
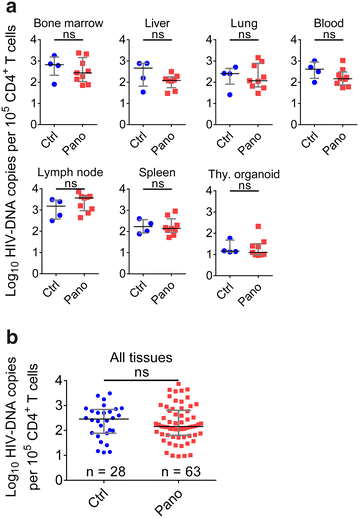
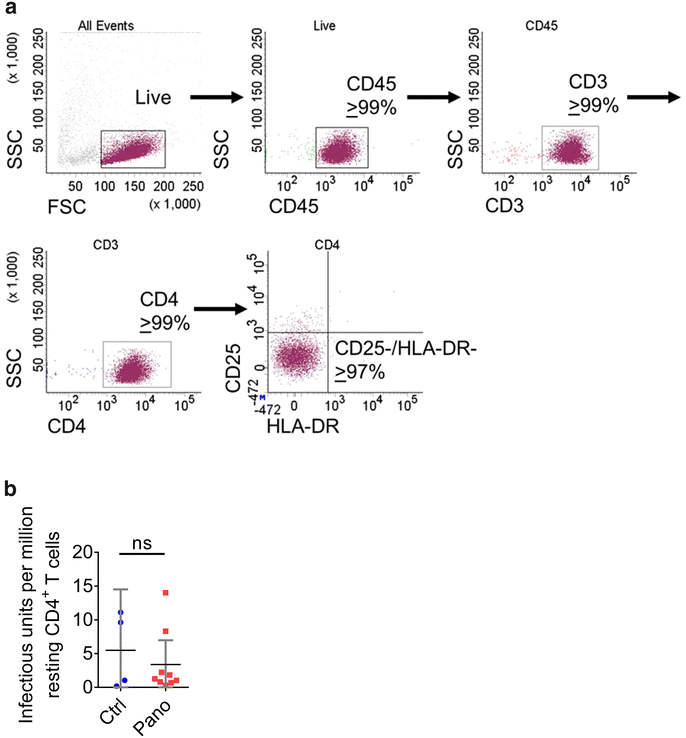
Results: Here, we have investigated the in vitro and systemic in vivo effect of panobinostat, a clinically relevant HDACi, on HIV latency. We showed that panobinostat induces histone acetylation in human PBMCs. Further, we showed that panobinostat induced HIV RNA expression and allowed the outgrowth of replication-competent virus ex vivo from resting CD4(+) T cells of HIV-infected patients on suppressive antiretroviral therapy (ART). Next, we demonstrated that panobinostat induced systemic histone acetylation in vivo in the tissues of BLT humanized mice. Finally, in HIV-infected, ART-suppressed BLT mice, we evaluated the effect of panobinostat on systemic cell-associated HIV RNA and DNA levels and the total frequency of latently infected resting CD4(+) T cells. Our data indicate that panobinostat treatment resulted in systemic increases in cellular levels of histone acetylation, a key biomarker for in vivo activity. However, panobinostat did not affect the levels of cell-associated HIV RNA, HIV DNA, or latently infected resting CD4(+) T cells.
Conclusion: We have demonstrated robust levels of systemic histone acetylation after panobinostat treatment of BLT humanized mice; and we did not observe a detectable change in the levels of cell-associated HIV RNA, HIV DNA, or latently infected resting CD4(+) T cells in HIV-infected, ART-suppressed BLT mice. These results are consistent with the modest effects noted in vitro and suggest that combination therapies may be necessary to reverse latency and enable clearance. Animal models will contribute to the progress towards an HIV cure.
Keywords: BLT; HIV; Histone acetylation; Humanized mice; Latency; Panobinostat.
Figures
Similar articles
-
Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat.J Virol. 2015 Oct;89(20):10176-89. doi: 10.1128/JVI.01484-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223643 Free PMC article. Clinical Trial.
-
Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients.Antimicrob Agents Chemother. 2015 Oct;59(10):5984-91. doi: 10.1128/AAC.01077-15. Epub 2015 Jul 13. Antimicrob Agents Chemother. 2015. PMID: 26169416 Free PMC article.
-
Analysis of the effect of HDAC inhibitors on the formation of the HIV reservoir.mBio. 2024 Sep 11;15(9):e0163224. doi: 10.1128/mbio.01632-24. Epub 2024 Aug 13. mBio. 2024. PMID: 39136440 Free PMC article.
-
Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV.Cell Host Microbe. 2018 Jan 10;23(1):14-26. doi: 10.1016/j.chom.2017.12.004. Cell Host Microbe. 2018. PMID: 29324227 Free PMC article. Review.
-
Therapeutic Approaches to Eradicate Latent HIV-1 in Resting CD4+ T Cells.Curr Top Med Chem. 2016;16(10):1191-7. doi: 10.2174/1568026615666150901114138. Curr Top Med Chem. 2016. PMID: 26324046 Review.
Cited by
-
Inducing Long-Term HIV-1 Latency in the TKO-BLT Mouse Model.Methods Mol Biol. 2022;2407:253-273. doi: 10.1007/978-1-0716-1871-4_18. Methods Mol Biol. 2022. PMID: 34985670
-
Impact of Tamoxifen on Vorinostat-Induced Human Immunodeficiency Virus Expression in Women on Antiretroviral Therapy: AIDS Clinical Trials Group A5366, The MOXIE Trial.Clin Infect Dis. 2022 Oct 12;75(8):1389-1396. doi: 10.1093/cid/ciac136. Clin Infect Dis. 2022. PMID: 35176755 Free PMC article. Clinical Trial.
-
Biogenesis of P-TEFb in CD4+ T cells to reverse HIV latency is mediated by protein kinase C (PKC)-independent signaling pathways.PLoS Pathog. 2021 Sep 16;17(9):e1009581. doi: 10.1371/journal.ppat.1009581. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34529720 Free PMC article.
-
Monitoring HIV DNA and cellular activation markers in HIV-infected humanized mice under cART.Virol J. 2018 Dec 17;15(1):191. doi: 10.1186/s12985-018-1101-9. Virol J. 2018. PMID: 30558630 Free PMC article.
-
Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL).Leukemia. 2019 Apr;33(4):844-862. doi: 10.1038/s41375-019-0388-x. Epub 2019 Jan 30. Leukemia. 2019. PMID: 30700842 Free PMC article. Review.
References
-
- Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

