Effects of Transforming Growth Factor Beta 1 in Cerebellar Development: Role in Synapse Formation
- PMID: 27199658
- PMCID: PMC4846658
- DOI: 10.3389/fncel.2016.00104
Effects of Transforming Growth Factor Beta 1 in Cerebellar Development: Role in Synapse Formation
Abstract
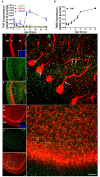
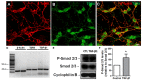
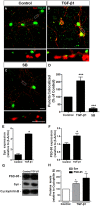
Granule cells (GC) are the most numerous glutamatergic neurons in the cerebellar cortex and represent almost half of the neurons of the central nervous system. Despite recent advances, the mechanisms of how the glutamatergic synapses are formed in the cerebellum remain unclear. Among the TGF-β family, TGF-beta 1 (TGF-β1) has been described as a synaptogenic molecule in invertebrates and in the vertebrate peripheral nervous system. A recent paper from our group demonstrated that TGF-β1 increases the excitatory synapse formation in cortical neurons. Here, we investigated the role of TGF-β1 in glutamatergic cerebellar neurons. We showed that the expression profile of TGF-β1 and its receptor, TβRII, in the cerebellum is consistent with a role in synapse formation in vitro and in vivo. It is low in the early postnatal days (P1-P9), increases after postnatal day 12 (P12), and remains high until adulthood (P30). We also found that granule neurons express the TGF-β receptor mRNA and protein, suggesting that they may be responsive to the synaptogenic effect of TGF-β1. Treatment of granular cell cultures with TGF-β1 increased the number of glutamatergic excitatory synapses by 100%, as shown by immunocytochemistry assays for presynaptic (synaptophysin) and post-synaptic (PSD-95) proteins. This effect was dependent on TβRI activation because addition of a pharmacological inhibitor of TGF-β, SB-431542, impaired the formation of synapses between granular neurons. Together, these findings suggest that TGF-β1 has a specific key function in the cerebellum through regulation of excitatory synapse formation between granule neurons.
Keywords: TGF-β1; cerebellum; development; excitatory synapse.
Figures

Similar articles
-
α-synuclein oligomers enhance astrocyte-induced synapse formation through TGF-β1 signaling in a Parkinson's disease model.J Neurochem. 2019 Jul;150(2):138-157. doi: 10.1111/jnc.14710. J Neurochem. 2019. PMID: 31009074
-
Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling.Glia. 2014 Dec;62(12):1917-31. doi: 10.1002/glia.22713. Epub 2014 Jul 10. Glia. 2014. PMID: 25042347
-
Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-beta1 pathways.J Neurochem. 2008 Jul;106(2):746-56. doi: 10.1111/j.1471-4159.2008.05428.x. Epub 2008 Apr 29. J Neurochem. 2008. PMID: 18419760
-
Multiple Phases of Climbing Fiber Synapse Elimination in the Developing Cerebellum.Cerebellum. 2018 Dec;17(6):722-734. doi: 10.1007/s12311-018-0964-z. Cerebellum. 2018. PMID: 30009357 Review.
-
Astrocytic control of neural circuit formation: highlights on TGF-beta signaling.Neurochem Int. 2014 Dec;78:18-27. doi: 10.1016/j.neuint.2014.07.008. Epub 2014 Aug 11. Neurochem Int. 2014. PMID: 25125369 Review.
Cited by
-
Potential role of TGFΒ and autophagy in early crebellum development.Biochem Biophys Rep. 2022 Oct 3;32:101358. doi: 10.1016/j.bbrep.2022.101358. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36213145 Free PMC article.
-
Tempora: Cell trajectory inference using time-series single-cell RNA sequencing data.PLoS Comput Biol. 2020 Sep 9;16(9):e1008205. doi: 10.1371/journal.pcbi.1008205. eCollection 2020 Sep. PLoS Comput Biol. 2020. PMID: 32903255 Free PMC article.
-
The Role of Astrocytes in the Development of the Cerebellum.Cerebellum. 2019 Dec;18(6):1017-1035. doi: 10.1007/s12311-019-01046-0. Cerebellum. 2019. PMID: 31218566 Review.
-
Telocytes' Role in Modulating Gut Motility Function and Development: Medical Hypotheses and Literature Review.Int J Mol Sci. 2022 Jun 24;23(13):7017. doi: 10.3390/ijms23137017. Int J Mol Sci. 2022. PMID: 35806023 Free PMC article. Review.
-
Flavonoid Hesperidin Induces Synapse Formation and Improves Memory Performance through the Astrocytic TGF-β1.Front Aging Neurosci. 2017 Jun 13;9:184. doi: 10.3389/fnagi.2017.00184. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28659786 Free PMC article.
References
-
- Adams N. C., Tomoda T., Cooper M., Dietz G., Hatten M. E. (2002). Mice that lack astrotactin have slowed neuronal migration. Development 129 965–972. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

