Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Endothelial Cells Accelerates Atherosclerosis in Mice
- PMID: 27199450
- PMCID: PMC4919153
- DOI: 10.1161/ATVBAHA.115.306670
Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Endothelial Cells Accelerates Atherosclerosis in Mice
Abstract
Objective: Plasma high-density lipoproteins have several putative antiatherogenic effects, including preservation of endothelial functions. This is thought to be mediated, in part, by the ability of high-density lipoproteins to promote cholesterol efflux from endothelial cells (ECs). The ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1) interact with high-density lipoproteins to promote cholesterol efflux from ECs. To determine the impact of endothelial cholesterol efflux pathways on atherogenesis, we prepared mice with endothelium-specific knockout of Abca1 and Abcg1.
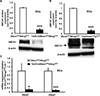
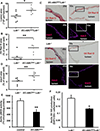
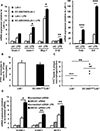
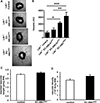
Approach and results: Generation of mice with EC-ABCA1 and ABCG1 deficiency required crossbreeding Abca1(fl/fl)Abcg1(fl/fl)Ldlr(-/-) mice with the Tie2Cre strain, followed by irradiation and transplantation of Abca1(fl/fl)Abcg1(fl/fl) bone marrow to abrogate the effects of macrophage ABCA1 and ABCG1 deficiency induced by Tie2Cre. After 20 to 22 weeks of Western-type diet, both single EC-Abca1 and Abcg1 deficiency increased atherosclerosis in the aortic root and whole aorta. Combined EC-Abca1/g1 deficiency caused a significant further increase in lesion area at both sites. EC-Abca1/g1 deficiency dramatically enhanced macrophage lipid accumulation in the branches of the aorta that are exposed to disturbed blood flow, decreased aortic endothelial NO synthase activity, and increased monocyte infiltration into the atherosclerotic plaque. Abca1/g1 deficiency enhanced lipopolysaccharide-induced inflammatory gene expression in mouse aortic ECs, which was recapitulated by ABCG1 deficiency in human aortic ECs.
Conclusions: These studies provide direct evidence that endothelial cholesterol efflux pathways mediated by ABCA1 and ABCG1 are nonredundant and atheroprotective, reflecting preservation of endothelial NO synthase activity and suppression of endothelial inflammation, especially in regions of disturbed arterial blood flow.
Keywords: ATP-binding cassette transporters; atherosclerosis; endothelium; hypercholesterolemia.
© 2016 American Heart Association, Inc.
Conflict of interest statement
A.R. Tall is a consultant to Amgen, Arisaph, and CSL. The other authors report no conflicts.
Figures



Similar articles
-
Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells.Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):279-84. doi: 10.1161/ATVBAHA.113.302781. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311381 Free PMC article.
-
Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis.Circulation. 2018 Aug 28;138(9):898-912. doi: 10.1161/CIRCULATIONAHA.117.032636. Circulation. 2018. PMID: 29588315 Free PMC article.
-
Melanocortin 1 Receptor Deficiency Promotes Atherosclerosis in Apolipoprotein E-/- Mice.Arterioscler Thromb Vasc Biol. 2018 Feb;38(2):313-323. doi: 10.1161/ATVBAHA.117.310418. Epub 2017 Dec 28. Arterioscler Thromb Vasc Biol. 2018. PMID: 29284608 Free PMC article.
-
ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis.Curr Drug Targets. 2011 May;12(5):647-60. doi: 10.2174/138945011795378522. Curr Drug Targets. 2011. PMID: 21039336 Review.
-
ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis.J Pharmacol Sci. 2022 Feb;148(2):197-203. doi: 10.1016/j.jphs.2021.11.005. Epub 2021 Dec 1. J Pharmacol Sci. 2022. PMID: 35063134 Review.
Cited by
-
Addressing dyslipidemic risk beyond LDL-cholesterol.J Clin Invest. 2022 Jan 4;132(1):e148559. doi: 10.1172/JCI148559. J Clin Invest. 2022. PMID: 34981790 Free PMC article. Review.
-
Diversity of Lipid Function in Atherogenesis: A Focus on Endothelial Mechanobiology.Int J Mol Sci. 2021 Oct 26;22(21):11545. doi: 10.3390/ijms222111545. Int J Mol Sci. 2021. PMID: 34768974 Free PMC article. Review.
-
AIBP, Angiogenesis, Hematopoiesis, and Atherogenesis.Curr Atheroscler Rep. 2020 Nov 24;23(1):1. doi: 10.1007/s11883-020-00899-9. Curr Atheroscler Rep. 2020. PMID: 33230630 Free PMC article. Review.
-
Macrophage-targeted nanomedicine for the diagnosis and management of atherosclerosis.Front Pharmacol. 2022 Sep 9;13:1000316. doi: 10.3389/fphar.2022.1000316. eCollection 2022. Front Pharmacol. 2022. PMID: 36160452 Free PMC article. Review.
-
Whole Blood Gene Expression Associated With Clinical Biological Age.J Gerontol A Biol Sci Med Sci. 2019 Jan 1;74(1):81-88. doi: 10.1093/gerona/gly164. J Gerontol A Biol Sci Med Sci. 2019. PMID: 30010802 Free PMC article.
References
-
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med. 1977;62:707–714. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

