IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer
- PMID: 27197187
- PMCID: PMC4891282
- DOI: 10.1158/0008-5472.CAN-15-2840
IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer
Abstract
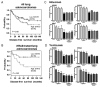
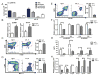
Activating mutations of K-ras are the most common oncogenic alterations found in lung cancer. Unfortunately, attempts to target K-ras-mutant lung tumors have thus far failed, clearly indicating the need for new approaches in patients with this molecular profile. We have previously shown NF-κB activation, release of IL6, and activation of its responsive transcription factor STAT3 in K-ras-mutant lung tumors, which was further amplified by the tumor-enhancing effect of chronic obstructive pulmonary disease (COPD)-type airway inflammation. These findings suggest an essential role for this inflammatory pathway in K-ras-mutant lung tumorigenesis and its enhancement by COPD. Therefore, here we blocked IL6 using a monoclonal anti-IL6 antibody in a K-ras-mutant mouse model of lung cancer in the absence or presence of COPD-type airway inflammation. IL6 blockade significantly inhibited lung cancer promotion, tumor cell-intrinsic STAT3 activation, tumor cell proliferation, and angiogenesis markers. Moreover, IL6 inhibition reduced expression of protumor type 2 molecules (arginase 1, Fizz 1, Mgl, and IDO), number of M2-type macrophages and granulocytic myeloid-derived suppressor cells, and protumor T-regulatory/Th17 cell responses. This was accompanied by increased expression of antitumor type 1 molecule (Nos2), and antitumor Th1/CD8 T-cell responses. Our study demonstrates that IL6 blockade not only has direct intrinsic inhibitory effect on tumor cells, but also reeducates the lung microenvironment toward an antitumor phenotype by altering the relative proportion between protumor and antitumor immune cells. This information introduces IL6 as a potential druggable target for prevention and treatment of K-ras-mutant lung tumors. Cancer Res; 76(11); 3189-99. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
Interleukin-6 Prevents the Initiation but Enhances the Progression of Lung Cancer.Cancer Res. 2015 Aug 15;75(16):3209-15. doi: 10.1158/0008-5472.CAN-14-3042. Epub 2015 Jun 29. Cancer Res. 2015. PMID: 26122841 Free PMC article.
-
IL22 Promotes Kras-Mutant Lung Cancer by Induction of a Protumor Immune Response and Protection of Stemness Properties.Cancer Immunol Res. 2018 Jul;6(7):788-797. doi: 10.1158/2326-6066.CIR-17-0655. Epub 2018 May 15. Cancer Immunol Res. 2018. PMID: 29764837 Free PMC article.
-
Expression of TLR9 in tumor-infiltrating mononuclear cells enhances angiogenesis and is associated with a worse survival in lung cancer.Int J Cancer. 2014 Feb 15;134(4):765-77. doi: 10.1002/ijc.28413. Epub 2013 Sep 4. Int J Cancer. 2014. PMID: 23913633
-
K-ras p21 expression and activity in lung and lung tumors.Exp Lung Res. 2000 Dec;26(8):659-71. doi: 10.1080/01902140150216747. Exp Lung Res. 2000. PMID: 11195463 Review.
-
Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment.Gen Thorac Cardiovasc Surg. 2014 Jul;62(7):415-21. doi: 10.1007/s11748-014-0386-x. Epub 2014 Mar 14. Gen Thorac Cardiovasc Surg. 2014. PMID: 24627306 Review.
Cited by
-
Valproic Acid-Like Compounds Enhance and Prolong the Radiotherapy Effect on Breast Cancer by Activating and Maintaining Anti-Tumor Immune Function.Front Immunol. 2021 May 12;12:646384. doi: 10.3389/fimmu.2021.646384. eCollection 2021. Front Immunol. 2021. PMID: 34054811 Free PMC article.
-
Pharmacological modulation of myeloid-derived suppressor cells to dampen inflammation.Front Immunol. 2022 Aug 30;13:933847. doi: 10.3389/fimmu.2022.933847. eCollection 2022. Front Immunol. 2022. PMID: 36110844 Free PMC article. Review.
-
If Virchow and Ehrlich Had Dreamt Together: What the Future Holds for KRAS-Mutant Lung Cancer.Int J Mol Sci. 2021 Mar 16;22(6):3025. doi: 10.3390/ijms22063025. Int J Mol Sci. 2021. PMID: 33809660 Free PMC article. Review.
-
Association of CXCL13 and Immune Cell Infiltration Signature in Clear Cell Renal Cell Carcinoma.Int J Med Sci. 2020 Jun 27;17(11):1610-1624. doi: 10.7150/ijms.46874. eCollection 2020. Int J Med Sci. 2020. PMID: 32669964 Free PMC article.
-
Characteristics of tumor microenvironment and novel immunotherapeutic strategies for non-small cell lung cancer.J Natl Cancer Cent. 2022 Oct 20;2(4):243-262. doi: 10.1016/j.jncc.2022.10.002. eCollection 2022 Dec. J Natl Cancer Cent. 2022. PMID: 39036549 Free PMC article. Review.
References
-
- Toll BA, Rojewski AM, Duncan LR, Latimer-Cheung AE, Fucito LM, Boyer JL, et al. “Quitting smoking will benefit your health”: the evolution of clinician messaging to encourage tobacco cessation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(2):301–9. - PMC - PubMed
-
- Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Archives of internal medicine. 2003;163(12):1475–80. - PubMed
-
- de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. American journal of respiratory and critical care medicine. 2015;191(3):285–91. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

