Three-Dimensional Environment Sustains Morphological Heterogeneity and Promotes Phenotypic Progression During Astrocyte Development
- PMID: 27193766
- PMCID: PMC4913501
- DOI: 10.1089/ten.TEA.2016.0103
Three-Dimensional Environment Sustains Morphological Heterogeneity and Promotes Phenotypic Progression During Astrocyte Development
Abstract
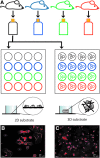
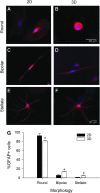
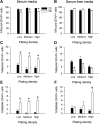
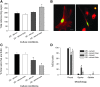
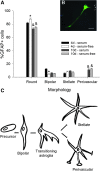
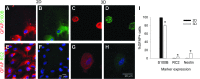
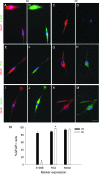
Astrocytes are critical for coordinating normal brain function by regulating brain metabolic homeostasis, synaptogenesis and neurotransmission, and blood-brain barrier permeability and maintenance. Dysregulation of normal astrocyte ontogeny contributes to neurodevelopmental and neurodegenerative disorders, epilepsies, and adverse responses to injury. To achieve these multiple essential roles, astrocyte phenotypes are regionally, morphologically, and functionally heterogeneous. Therefore, the best regenerative medicine strategies may require selective production of distinct astrocyte subpopulations at defined maturation levels. However, little is known about the mechanisms that direct astrocyte diversity or whether heterogeneity is represented in biomaterials. In vitro studies report lack of normal morphologies and overrepresentation of the glial scar type of reactive astrocyte morphology and expression of markers, questioning how well the in vitro astrocytes represent glia in vivo and whether in vitro tissue engineering methods are suitable for regenerative medicine applications. Our previous work with neurons suggests that the three-dimensional (3D) environment, when compared with standard two-dimensional (2D) substrate, yields cellular and molecular behaviors that more closely approximately normal ontogeny. To specifically study the effects of dimensionality, we used purified glial fibrillary acidic protein (GFAP)-expressing primary cerebral cortical astrocyte cultures from single pups and characterized the cellular maturation profiles in 2D and 3D milieu. We identified four morphological groups in vitro: round, bipolar, stellate, and putative perivascular. In the 3D hydrogel culture environment, postnatal astrocytes transitioned from a population of nearly all round cells and very few bipolar cells toward a population with significant fractions of round, stellate, and putative perivascular cells within a few days, following the in vivo ontogeny. In 2D, however, the population shift from round and bipolar to stellate and perivascular was rarely observed. The transition to distinct cellular morphologies in 3D corresponded to the in vivo expression of phenotypic markers, supporting the generation of mature heterogeneous glial populations in vitro. This study presents quantitative data supporting that 3D culture is critical for sustaining the heterogeneity of astrocytes in vitro and for generating a representation of the in vivo portfolio of heterogeneous populations of astrocytes required for therapeutic interventions in neurodevelopmental disorders, epilepsy, and brain injury.
Figures
Similar articles
-
A cortical astrocyte subpopulation inhibits axon growth in vitro and in vivo.Mol Med Rep. 2015 Aug;12(2):2598-606. doi: 10.3892/mmr.2015.3702. Epub 2015 Apr 29. Mol Med Rep. 2015. PMID: 25936767 Free PMC article.
-
Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes.Glia. 2006 Feb;53(3):277-93. doi: 10.1002/glia.20281. Glia. 2006. PMID: 16267834
-
In vitro induction of in vivo-relevant stellate astrocytes in 3D brain-derived, decellularized extracellular matrices.Acta Biomater. 2023 Dec;172:218-233. doi: 10.1016/j.actbio.2023.09.046. Epub 2023 Oct 1. Acta Biomater. 2023. PMID: 37788738
-
Beyond the GFAP-Astrocyte Protein Markers in the Brain.Biomolecules. 2021 Sep 14;11(9):1361. doi: 10.3390/biom11091361. Biomolecules. 2021. PMID: 34572572 Free PMC article. Review.
-
Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans.Glia. 2021 Sep;69(9):2077-2099. doi: 10.1002/glia.23994. Epub 2021 Mar 18. Glia. 2021. PMID: 33734498 Review.
Cited by
-
In Vivo Imaging of Allografted Glial-Restricted Progenitor Cell Survival and Hydrogel Scaffold Biodegradation.ACS Appl Mater Interfaces. 2021 May 26;13(20):23423-23437. doi: 10.1021/acsami.1c03415. Epub 2021 May 12. ACS Appl Mater Interfaces. 2021. PMID: 33978398 Free PMC article.
-
Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models.Materials (Basel). 2019 Oct 1;12(19):3218. doi: 10.3390/ma12193218. Materials (Basel). 2019. PMID: 31581436 Free PMC article. Review.
-
Simple Design for Membrane-Free Microphysiological Systems to Model the Blood-Tissue Barriers.bioRxiv [Preprint]. 2023 Oct 23:2023.10.20.563328. doi: 10.1101/2023.10.20.563328. bioRxiv. 2023. PMID: 37961220 Free PMC article. Preprint.
-
Impact of perfusion on neuronal development in human derived neuronal networks.APL Bioeng. 2024 Oct 1;8(4):046102. doi: 10.1063/5.0221911. eCollection 2024 Dec. APL Bioeng. 2024. PMID: 39364213 Free PMC article.
-
An Overview of Multiple Sclerosis In Vitro Models.Int J Mol Sci. 2024 Jul 16;25(14):7759. doi: 10.3390/ijms25147759. Int J Mol Sci. 2024. PMID: 39063001 Free PMC article. Review.
References
-
- Molofsky A.V., and Deneen B. Astrocyte development: a guide for the perplexed. Glia 63, 1320, 2015 - PubMed
-
- Kimelberg H. Astrocyte heterogeneity or homogeneity? In: Parpura V., Haydon P.G., eds. Astrocytes in (Patho)physiology of the Nervous System. New York, NY: Springer, 2009, p. 1.
-
- Matyash V., and Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63, 2, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

