Calcineurin in a Crowded World
- PMID: 27187005
- PMCID: PMC4900146
- DOI: 10.1021/acs.biochem.6b00059
Calcineurin in a Crowded World
Abstract
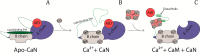
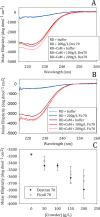
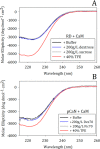
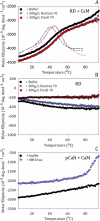
Calcineurin is a Ser/Thr phosphatase that is important for key biological processes, including immune system activation. We previously identified a region in the intrinsically disordered regulatory domain of calcineurin that forms a critical amphipathic α-helix (the "distal helix") that is required for complete activation of calcineurin. This distal helix was shown to have a Tm close to that of human body temperature. Because the Tm was determined in dilute buffer, we hypothesized that other factors inherent to a cellular environment might modulate the stability of the distal helix. One such factor that contributes to stability in other proteins is macromolecular crowding. The cell cytoplasm is comprised of up to 400 g/L protein, lipids, nucleic acids, and other compounds. We hypothesize that the presence of such crowders could increase the thermal stability of the distal helix and thus lead to a more robust activation of calcineurin in vivo. Using biophysical and biochemical approaches, we show that the distal helix of calcineurin is indeed stabilized when crowded by the synthetic polymers dextran 70 and ficoll 70, and that this stabilization of the distal helix increases the activity of calcineurin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Calcineurin.Cell Commun Signal. 2020 Aug 28;18(1):137. doi: 10.1186/s12964-020-00636-4. Cell Commun Signal. 2020. PMID: 32859215 Free PMC article. Review.
-
The distal helix in the regulatory domain of calcineurin is important for domain stability and enzyme function.Biochemistry. 2013 Dec 3;52(48):8643-51. doi: 10.1021/bi400483a. Epub 2013 Nov 15. Biochemistry. 2013. PMID: 24191726
-
Calmodulin-Calcineurin Interaction beyond the Calmodulin-Binding Region Contributes to Calcineurin Activation.Biochemistry. 2019 Oct 1;58(39):4070-4085. doi: 10.1021/acs.biochem.9b00626. Epub 2019 Sep 19. Biochemistry. 2019. PMID: 31483613 Free PMC article.
-
Soft interactions and volume exclusion by polymeric crowders can stabilize or destabilize transient structure in disordered proteins depending on polymer concentration.Proteins. 2017 Aug;85(8):1468-1479. doi: 10.1002/prot.25307. Epub 2017 May 16. Proteins. 2017. PMID: 28425679
-
Regulation of the calmodulin-stimulated protein phosphatase, calcineurin.J Biol Chem. 1998 May 29;273(22):13367-70. doi: 10.1074/jbc.273.22.13367. J Biol Chem. 1998. PMID: 9593662 Review. No abstract available.
Cited by
-
Large cosolutes, small cosolutes, and dihydrofolate reductase activity.Protein Sci. 2017 Dec;26(12):2417-2425. doi: 10.1002/pro.3316. Epub 2017 Nov 17. Protein Sci. 2017. PMID: 28971539 Free PMC article.
-
Features of molecular recognition of intrinsically disordered proteins via coupled folding and binding.Protein Sci. 2019 Nov;28(11):1952-1965. doi: 10.1002/pro.3718. Epub 2019 Sep 4. Protein Sci. 2019. PMID: 31441158 Free PMC article. Review.
-
Calcineurin B inhibits calcium oxalate crystallization, growth and aggregation via its high calcium-affinity property.Comput Struct Biotechnol J. 2023 Jul 31;21:3854-3864. doi: 10.1016/j.csbj.2023.07.038. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37593722 Free PMC article.
-
Calcineurin.Cell Commun Signal. 2020 Aug 28;18(1):137. doi: 10.1186/s12964-020-00636-4. Cell Commun Signal. 2020. PMID: 32859215 Free PMC article. Review.
-
Cleavage-Resistant Protein Labeling With Hydrophilic Trityl Enables Distance Measurements In-Cell.J Phys Chem B. 2021 May 27;125(20):5265-5274. doi: 10.1021/acs.jpcb.1c02371. Epub 2021 May 13. J Phys Chem B. 2021. PMID: 33983738 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

