α-Amanitin Restrains Cancer Relapse from Drug-Tolerant Cell Subpopulations via TAF15
- PMID: 27181033
- PMCID: PMC4867652
- DOI: 10.1038/srep25895
α-Amanitin Restrains Cancer Relapse from Drug-Tolerant Cell Subpopulations via TAF15
Abstract
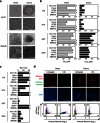
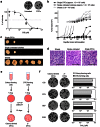
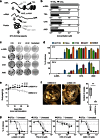
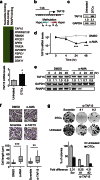
Cancer relapse occurs with substantial frequency even after treatment with curative intent. Here we studied drug-tolerant colonies (DTCs), which are subpopulations of cancer cells that survive in the presence of drugs. Proteomic characterization of DTCs identified stemness- and epithelial-dominant subpopulations, but functional screening suggested that DTC formation was regulated at the transcriptional level independent from protein expression patterns. We consistently found that α-amanitin, an RNA polymerase II (RNAPII) inhibitor, effectively inhibited DTCs by suppressing TAF15 expression, which binds to RNA to modulate transcription and RNA processing. Sequential administration of α-amanitin and cisplatin extended overall survival in a cancer-relapse mouse model, namely peritonitis carcinomatosa. Therefore, post-treatment cancer relapse may occur through non-distinct subpopulations and may be effectively prevented by α-amanitin to disrupt transcriptional machinery, including TAF15.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma.J Natl Cancer Inst. 2012 Apr 18;104(8):622-34. doi: 10.1093/jnci/djs140. Epub 2012 Mar 27. J Natl Cancer Inst. 2012. PMID: 22457476
-
Silencing GOLPH3 gene expression reverses resistance to cisplatin in HT29 colon cancer cells via multiple signaling pathways.Int J Oncol. 2018 Sep;53(3):1183-1192. doi: 10.3892/ijo.2018.4471. Epub 2018 Jul 5. Int J Oncol. 2018. PMID: 30015866
-
Domains involved in TAF15 subcellular localisation: dependence on cell type and ongoing transcription.Gene. 2012 Sep 15;506(2):331-8. doi: 10.1016/j.gene.2012.06.088. Epub 2012 Jul 3. Gene. 2012. PMID: 22771914
-
The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells.Cell Oncol (Dordr). 2017 Aug;40(4):367-378. doi: 10.1007/s13402-017-0328-6. Epub 2017 Jun 7. Cell Oncol (Dordr). 2017. PMID: 28593575
-
TLS, EWS and TAF15: a model for transcriptional integration of gene expression.Brief Funct Genomic Proteomic. 2006 Mar;5(1):8-14. doi: 10.1093/bfgp/ell015. Epub 2006 Feb 23. Brief Funct Genomic Proteomic. 2006. PMID: 16769671 Review.
Cited by
-
Meeting key synthetic challenges in amanitin synthesis with a new cytotoxic analog: 5'-hydroxy-6'-deoxy-amanitin.Chem Sci. 2020 Oct 15;11(43):11927-11935. doi: 10.1039/d0sc04150e. Chem Sci. 2020. PMID: 34094418 Free PMC article.
-
Differential Expression of Amanitin Biosynthetic Genes and Novel Cyclic Peptides in Amanita molliuscula.J Fungi (Basel). 2021 May 14;7(5):384. doi: 10.3390/jof7050384. J Fungi (Basel). 2021. PMID: 34069263 Free PMC article.
-
Downregulation of LncRNA DARS-AS1 Inhibits the Tumorigenesis of Cervical Cancer via Inhibition of IGF2BP3.Onco Targets Ther. 2021 Feb 25;14:1331-1340. doi: 10.2147/OTT.S274623. eCollection 2021. Onco Targets Ther. 2021. PMID: 33658798 Free PMC article.
-
TAF15 promotes cell proliferation, migration and invasion of gastric cancer via activation of the RAF1/MEK/ERK signalling pathway.Sci Rep. 2023 Apr 10;13(1):5846. doi: 10.1038/s41598-023-31959-0. Sci Rep. 2023. PMID: 37037864 Free PMC article.
-
Novel Cyclic Peptides from Lethal Amanita Mushrooms through a Genome-Guided Approach.J Fungi (Basel). 2021 Mar 11;7(3):204. doi: 10.3390/jof7030204. J Fungi (Basel). 2021. PMID: 33799506 Free PMC article.
References
-
- Sakuramoto S. et al.. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357, 1810–1820 (2007). - PubMed
-
- Paoletti X. et al.. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303, 1729–1737 (2010). - PubMed
-
- O’Connell M. J. et al.. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 331, 502–507 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

