2-Deoxy-d-glucose Suppresses the In Vivo Antitumor Efficacy of Erlotinib in Head and Neck Squamous Cell Carcinoma Cells
- PMID: 27178822
- PMCID: PMC5282972
- DOI: 10.3727/096504016X14586627440192
2-Deoxy-d-glucose Suppresses the In Vivo Antitumor Efficacy of Erlotinib in Head and Neck Squamous Cell Carcinoma Cells
Abstract
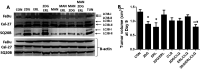
Poor tumor response to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is a significant challenge for effective treatment of head and neck squamous cell carcinoma (HNSCC). Therefore, strategies that may increase tumor response to EGFR TKIs are warranted in order to improve HNSCC patient treatment and overall survival. HNSCC tumors are highly glycolytic, and increased EGFR signaling has been found to promote glucose metabolism through various mechanisms. We have previously shown that inhibition of glycolysis with 2-deoxy-d-glucose (2DG) significantly enhanced the antitumor effects of cisplatin and radiation, which are commonly used to treat HNSCC. The goal of the current studies is to determine if 2DG will enhance the antitumor activity of the EGFR TKI erlotinib in HNSCC. Erlotinib transiently suppressed glucose consumption accompanied by alterations in pyruvate kinase M2 (PKM2) expression. 2DG enhanced the cytotoxic effect of erlotinib in vitro but reversed the antitumor effect of erlotinib in vivo. 2DG altered the N-glycosylation status of EGFR and induced the endoplasmic reticulum (ER) stress markers CHOP and BiP in vitro. Additionally, the effects of 2DG + erlotinib on cytotoxicity and ER stress in vitro were reversed by mannose but not glucose or antioxidant enzymes. Lastly, the protective effect of 2DG on erlotinib-induced cytotoxicity in vivo was reversed by chloroquine. Altogether, 2DG suppressed the antitumor efficacy of erlotinib in a HNSCC xenograft mouse model, which may be due to increased cytoprotective autophagy mediated by ER stress activation.
Conflict of interest statement
The authors have no conflict of interest.
Figures

Similar articles
-
Elevated RET expression enhances EGFR activation and mediates EGFR inhibitor resistance in head and neck squamous cell carcinoma.Cancer Lett. 2016 Jul 10;377(1):1-10. doi: 10.1016/j.canlet.2016.04.023. Epub 2016 Apr 18. Cancer Lett. 2016. PMID: 27090738
-
MAPK1E322K mutation increases head and neck squamous cell carcinoma sensitivity to erlotinib through enhanced secretion of amphiregulin.Oncotarget. 2016 Apr 26;7(17):23300-11. doi: 10.18632/oncotarget.8188. Oncotarget. 2016. PMID: 27004400 Free PMC article.
-
Preclinical modeling of EGFR inhibitor resistance in head and neck cancer.Cancer Biol Ther. 2012 Aug;13(10):935-45. doi: 10.4161/cbt.20846. Epub 2012 Aug 1. Cancer Biol Ther. 2012. PMID: 22785204 Free PMC article.
-
Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy.Pathol Res Pract. 2011 Jun 15;207(6):337-42. doi: 10.1016/j.prp.2011.03.002. Epub 2011 Apr 29. Pathol Res Pract. 2011. PMID: 21531084 Review.
-
Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC).Cancer Treat Rev. 2014 May;40(4):567-77. doi: 10.1016/j.ctrv.2013.10.002. Epub 2013 Oct 12. Cancer Treat Rev. 2014. PMID: 24216225 Review.
Cited by
-
Advantages of the Combinatorial Molecular Targeted Therapy of Head and Neck Cancer-A Step before Anakoinosis-Based Personalized Treatment.Cancers (Basel). 2023 Aug 24;15(17):4247. doi: 10.3390/cancers15174247. Cancers (Basel). 2023. PMID: 37686523 Free PMC article. Review.
-
SG2NA is a regulator of endoplasmic reticulum (ER) homeostasis as its depletion leads to ER stress.Cell Stress Chaperones. 2017 Nov;22(6):853-866. doi: 10.1007/s12192-017-0816-7. Epub 2017 Jun 21. Cell Stress Chaperones. 2017. PMID: 28634818 Free PMC article.
-
Metabolic Reprogramming of Thyroid Cancer Cells and Crosstalk in Their Microenvironment.Front Oncol. 2021 Dec 2;11:773028. doi: 10.3389/fonc.2021.773028. eCollection 2021. Front Oncol. 2021. PMID: 34926283 Free PMC article. Review.
-
Targeting the unfolded protein response in head and neck and oral cavity cancers.Exp Cell Res. 2019 Sep 1;382(1):111386. doi: 10.1016/j.yexcr.2019.04.007. Epub 2019 May 7. Exp Cell Res. 2019. PMID: 31075256 Free PMC article. Review.
-
Laminar Flow on Endothelial Cells Suppresses eNOS O-GlcNAcylation to Promote eNOS Activity.Circ Res. 2021 Nov 12;129(11):1054-1066. doi: 10.1161/CIRCRESAHA.121.318982. Epub 2021 Oct 4. Circ Res. 2021. PMID: 34605247 Free PMC article.
References
-
- Ang K. K.; Berkey B. A.; Tu X.; Zhang H. Z.; Katz R.; Hammond E. H.; Fu K. K.; Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62:7350–7356; 2002. - PubMed
-
- Grandis J. R.; Tweardy D. J. TGF-alpha and EGFR in head and neck cancer. J. Cell Biochem. Suppl. 17F:188–191; 1993. - PubMed
-
- Loeffler-Ragg J.; Schwentner I.; Sprinzl G. M.; Zwierzina H. EGFR inhibition as a therapy for head and neck squamous cell carcinoma. Expert Opin. Investig. Drugs 17:1517–1531; 2008. - PubMed
-
- Lynch T. J.; Bell D. W.; Sordella R.; Gurubhagavatula S.; Okimoto R. A.; Brannigan B. W.; Harris P. L.; Haserlat S. M.; Supko J. G.; Haluska F. G.; Louis D. N.; Christiani D. C.; Settleman J.; Haber D. A. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350:2129–2139; 2004. - PubMed
-
- Sequist L. V.; Bell D. W.; Lynch T. J.; Haber D. A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 25:587–595; 2007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
