Treatment interruption after 2-year antiretroviral treatment initiated during acute/early HIV in infancy
- PMID: 27177316
- PMCID: PMC5014697
- DOI: 10.1097/QAD.0000000000001158
Treatment interruption after 2-year antiretroviral treatment initiated during acute/early HIV in infancy
Abstract
Objective: Treatment interruption has been well tolerated and durable in some pediatric studies but none have compared treatment interruption with continued antiretroviral treatment (ART) following ART initiation in early HIV. The objective of this study was to compare outcomes in treatment interruption versus continued ART among early-treated infants.
Design: Randomized trial (OPH-03; NCT00428116).
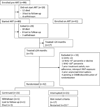
Methods: The trial included HIV-infected infants who initiated ART at less than 13 months of age, received ART for 24 months, and, if eligible (CD4% >25%, normal growth), were randomized to treatment interruption versus continued ART. Children in the treatment interruption group restarted ART if they met WHO ART-eligibility criteria. During 18-months postrandomization, primary outcomes were incidence of serious adverse events and growth. CD4%, viral load, morbidity, and growth were compared.
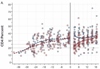
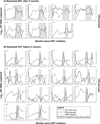
Results: Of 140 infants enrolled, 121 started ART, of whom 75 completed at least 24 months ART and 42 were randomized (21 per arm). ART was initiated at median age 5 months and randomization at 30 months. Among 21 treatment interruption children, 14 met ART restart criteria within 3 months. Randomization was discontinued by Data and Safety Monitoring Board due to low treatment interruption durability. At 18 months postrandomization, growth and serious adverse events were comparable between arms; hypercholesteremia incidence was higher in the continued arm (P = 0.03). CD4% and viral load did not differ between arms [CD4% 35% and median viral load undetectable (<150 copies/ml) in both arms, P = 0.9 for each comparison]. No infants had post-treatment viral control.
Conclusion: Short treatment interruption did not compromise 18-month CD4%, viral control, growth, or morbidity compared with continued ART among infants who started ART in early HIV infection.
Figures




Comment in
-
Treatment interruption after early-treated perinatal HIV-1 infection.AIDS. 2016 Sep 24;30(15):2381-3. doi: 10.1097/QAD.0000000000001226. AIDS. 2016. PMID: 27603162 No abstract available.
Similar articles
-
Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial.Lancet. 2013 Nov 9;382(9904):1555-63. doi: 10.1016/S0140-6736(13)61409-9. Lancet. 2013. PMID: 24209829 Free PMC article. Clinical Trial.
-
HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report.Lancet HIV. 2016 Jan;3(1):e49-54. doi: 10.1016/S2352-3018(15)00232-5. Epub 2015 Dec 9. Lancet HIV. 2016. PMID: 26762993
-
HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy.Antivir Ther. 2012;17(6):1001-9. doi: 10.3851/IMP2273. Epub 2012 Aug 6. Antivir Ther. 2012. PMID: 22865544
-
[Consensus document of Gesida and Spanish Secretariat for the National Plan on AIDS (SPNS) regarding combined antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2012)].Enferm Infecc Microbiol Clin. 2012 Jun;30(6):e1-89. doi: 10.1016/j.eimc.2012.03.006. Epub 2012 May 23. Enferm Infecc Microbiol Clin. 2012. PMID: 22633764 Spanish.
-
[Recommendations from the GESIDA/Spanish AIDS Plan regarding antiretroviral treatment in adults with human immunodeficiency virus infection (update February 2009)].Enferm Infecc Microbiol Clin. 2009 Apr;27(4):222-35. doi: 10.1016/j.eimc.2008.11.002. Epub 2009 Feb 26. Enferm Infecc Microbiol Clin. 2009. PMID: 19246124 Spanish.
Cited by
-
Impact of the time to achieve viral control on the dynamics of circulating HIV-1 reservoir in vertically infected children with long-term sustained virological suppression: A longitudinal study.PLoS One. 2018 Oct 23;13(10):e0205579. doi: 10.1371/journal.pone.0205579. eCollection 2018. PLoS One. 2018. PMID: 30352067 Free PMC article.
-
Association Between Cytomegalovirus and Epstein-Barr Virus Viremia And Human Immunodeficiency Virus DNA Levels in the Reservoir of Kenyan Infants Receiving Antiretroviral Therapy.J Infect Dis. 2021 Jun 4;223(11):1923-1927. doi: 10.1093/infdis/jiaa640. J Infect Dis. 2021. PMID: 33064809 Free PMC article.
-
Learning From the Exceptions: HIV Remission in Post-treatment Controllers.Front Immunol. 2019 Jul 24;10:1749. doi: 10.3389/fimmu.2019.01749. eCollection 2019. Front Immunol. 2019. PMID: 31396237 Free PMC article. Review.
-
Decay of HIV DNA in the Reservoir and the Impact of Short Treatment Interruption in Kenyan Infants.Open Forum Infect Dis. 2017 Dec 9;5(1):ofx268. doi: 10.1093/ofid/ofx268. eCollection 2018 Jan. Open Forum Infect Dis. 2017. PMID: 29354661 Free PMC article.
-
Brief Report: Group-Based Trajectory Modeling to Determine Long-Term HIV Viral Load Trends Among Children With HIV in Kenya.J Acquir Immune Defic Syndr. 2024 Aug 1;96(4):311-317. doi: 10.1097/QAI.0000000000003439. J Acquir Immune Defic Syndr. 2024. PMID: 39287566 Clinical Trial.
References
-
- Alexander L, Cuchura L, Simpson BJ, Andiman WA. Virologic and host characteristics of human immunodeficiency virus type 1-infected pediatric long term survivors. The Pediatric Infectious Disease Journal. 2006;25:135–141. - PubMed
-
- Frange P, Faye A, Avettand-Fenoel V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3:e49–e54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

