Your neighbours matter - non-autonomous control of apoptosis in development and disease
- PMID: 27177021
- PMCID: PMC4946894
- DOI: 10.1038/cdd.2016.41
Your neighbours matter - non-autonomous control of apoptosis in development and disease
Abstract
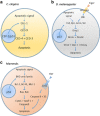
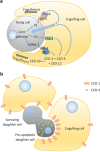
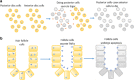
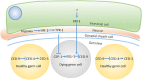
Traditionally, the regulation of apoptosis has been thought of as an autonomous process in which the dying cell dictates its own demise. However, emerging studies in genetically tractable multicellular organisms, such as Caenorhabditis elegans and Drosophila, have revealed that death is often a communal event. Here, we review the current literature on non-autonomous mechanisms governing apoptosis in multiple cellular contexts. The importance of the cellular community in dictating the funeral arrangements of apoptotic cells has profound implications in development and disease.
Figures
Similar articles
-
The genetics of hiding the corpse: engulfment and degradation of apoptotic cells in C. elegans and D. melanogaster.Curr Top Dev Biol. 2004;63:91-143. doi: 10.1016/S0070-2153(04)63004-3. Curr Top Dev Biol. 2004. PMID: 15536015 Review. No abstract available.
-
Autonomous and non-autonomous roles of DNase II during cell death in C. elegans embryos.Biosci Rep. 2015 Apr 27;35(3):e00203. doi: 10.1042/BSR20150055. Biosci Rep. 2015. PMID: 26182365 Free PMC article.
-
NF-Y in invertebrates.Biochim Biophys Acta Gene Regul Mech. 2017 May;1860(5):630-635. doi: 10.1016/j.bbagrm.2016.10.008. Epub 2016 Oct 26. Biochim Biophys Acta Gene Regul Mech. 2017. PMID: 27793714 Review.
-
Proapoptotic activity of Caenorhabditis elegans CED-4 protein in Drosophila: implicated mechanisms for caspase activation.Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):145-50. doi: 10.1073/pnas.96.1.145. Proc Natl Acad Sci U S A. 1999. PMID: 9874786 Free PMC article.
-
Both the apoptotic suicide pathway and phagocytosis are required for a programmed cell death in Caenorhabditis elegans.BMC Biol. 2016 May 16;14:39. doi: 10.1186/s12915-016-0262-5. BMC Biol. 2016. PMID: 27185172 Free PMC article.
Cited by
-
Hydroxyurea Exposure and Development of the Cerebellar External Granular Layer: Effects on Granule Cell Precursors, Bergmann Glial and Microglial Cells.Neurotox Res. 2019 Feb;35(2):387-400. doi: 10.1007/s12640-018-9964-5. Epub 2018 Oct 1. Neurotox Res. 2019. PMID: 30276718
-
Impact of inhibitor of apoptosis proteins on immune modulation and inflammation.Immunol Cell Biol. 2017 Mar;95(3):236-243. doi: 10.1038/icb.2016.101. Epub 2016 Oct 7. Immunol Cell Biol. 2017. PMID: 27713393 Review.
-
Ursolic Acid-Induced Apoptosis via Regulation of the PI3K/Akt and MAPK Signaling Pathways in Huh-7 Cells.Molecules. 2018 Aug 13;23(8):2016. doi: 10.3390/molecules23082016. Molecules. 2018. PMID: 30104508 Free PMC article.
-
Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors.Int J Mol Sci. 2016 Dec 20;17(12):2144. doi: 10.3390/ijms17122144. Int J Mol Sci. 2016. PMID: 27999411 Free PMC article. Review.
-
Control of non-apoptotic nurse cell death by engulfment genes in Drosophila.Fly (Austin). 2017 Apr 3;11(2):104-111. doi: 10.1080/19336934.2016.1238993. Epub 2016 Sep 29. Fly (Austin). 2017. PMID: 27686122 Free PMC article.
References
-
- Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Frohlich K. Apoptosis in yeast. Curr Opin Microbiol 2004; 7: 655–660. - PubMed
-
- Lettre G, Hengartner M. Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol 2006; 7: 97–108. - PubMed
-
- Hipfner D, Cohen S. Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol 2004; 5: 805–815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

