Derivation of Porcine Embryonic Stem-Like Cells from In Vitro-Produced Blastocyst-Stage Embryos
- PMID: 27173828
- PMCID: PMC4865852
- DOI: 10.1038/srep25838
Derivation of Porcine Embryonic Stem-Like Cells from In Vitro-Produced Blastocyst-Stage Embryos
Abstract
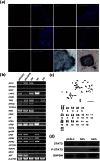
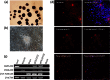
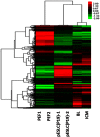
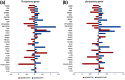
Efficient isolation of embryonic stem (ES) cells from pre-implantation porcine embryos has remained a challenge. Here, we describe the derivation of porcine embryonic stem-like cells (pESLCs) by seeding the isolated inner cell mass (ICM) from in vitro-produced porcine blastocyst into α-MEM with basic fibroblast growth factor (bFGF). The pESL cells kept the normal karyotype and displayed flatten clones, similar in phenotype to human embryonic stem cells (hES cells) and rodent epiblast stem cells. These cells exhibited alkaline phosphatase (AP) activity and expressed pluripotency markers such as OCT4, NANOG, SOX2, SSEA-4, TRA-1-60, and TRA-1-81 as determined by both immunofluorescence and RT-PCR. Additionally, these cells formed embryoid body (EB), teratomas and also differentiated into 3 germ layers in vitro and in vivo. Microarray analysis showed the expression of the pluripotency markers, PODXL, REX1, SOX2, KLF5 and NR6A1, was significantly higher compared with porcine embryonic fibroblasts (PEF), but expression of OCT4, TBX3, REX1, LIN28A and DPPA5, was lower compared to the whole blastocysts or ICM of blastocyst. Our results showed that porcine embryonic stem-like cells can be established from in vitro-produced blastocyst-stage embryos, which promote porcine naive ES cells to be established.
Figures


Similar articles
-
Generation and characterization of embryonic stem-like cell lines derived from in vitro fertilization Buffalo (Bubalus bubalis) embryos.Reprod Domest Anim. 2010 Feb;45(1):122-8. doi: 10.1111/j.1439-0531.2008.01268.x. Epub 2008 Dec 22. Reprod Domest Anim. 2010. PMID: 19144015
-
Development of human embryonic stem cell derivation.J Med Assoc Thai. 2009 Apr;92(4):443-50. J Med Assoc Thai. 2009. PMID: 19374291
-
Analysis of co-expression of OCT4, NANOG and SOX2 in pluripotent cells of the porcine embryo, in vivo and in vitro.Theriogenology. 2011 Feb;75(3):513-26. doi: 10.1016/j.theriogenology.2010.09.019. Epub 2010 Nov 12. Theriogenology. 2011. PMID: 21074831
-
Porcine embryonic stem cells: a possible source for cell replacement therapy.Stem Cell Rev. 2008 Dec;4(4):275-82. doi: 10.1007/s12015-008-9040-2. Stem Cell Rev. 2008. PMID: 18770051 Review.
-
Nonviable human pre-implantation embryos as a source of stem cells for research and potential therapy.Stem Cell Rev. 2005 Dec;1(4):337-43. doi: 10.1385/SCR:1:4:337. Stem Cell Rev. 2005. PMID: 17142877 Review.
Cited by
-
Tetraploid embryo aggregation produces high-quality blastocysts with an increased trophectoderm in pigs.Front Cell Dev Biol. 2023 Nov 16;11:1239448. doi: 10.3389/fcell.2023.1239448. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033873 Free PMC article.
-
Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States.Int J Mol Sci. 2021 Nov 29;22(23):12918. doi: 10.3390/ijms222312918. Int J Mol Sci. 2021. PMID: 34884722 Free PMC article.
-
Modeling lethal X-linked genetic disorders in pigs with ensured fertility.Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):708-713. doi: 10.1073/pnas.1715940115. Epub 2018 Jan 8. Proc Natl Acad Sci U S A. 2018. PMID: 29311328 Free PMC article.
-
Generation and characterization of stable pig pregastrulation epiblast stem cell lines.Cell Res. 2022 Apr;32(4):383-400. doi: 10.1038/s41422-021-00592-9. Epub 2021 Nov 30. Cell Res. 2022. PMID: 34848870 Free PMC article.
-
Establishment of porcine and human expanded potential stem cells.Nat Cell Biol. 2019 Jun;21(6):687-699. doi: 10.1038/s41556-019-0333-2. Epub 2019 Jun 3. Nat Cell Biol. 2019. PMID: 31160711 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

