Dual function of cTAGE5 in collagen export from the endoplasmic reticulum
- PMID: 27170179
- PMCID: PMC4927275
- DOI: 10.1091/mbc.E16-03-0180
Dual function of cTAGE5 in collagen export from the endoplasmic reticulum
Abstract
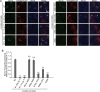
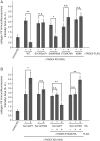
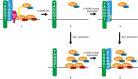
Two independent functions of cTAGE5 have been reported in collagen VII export from the endoplasmic reticulum (ER). cTAGE5 not only forms a cargo receptor complex with TANGO1, but it also acts as a scaffold to recruit Sec12, a guanine-nucleotide exchange factor for Sar1 GTPase, to ER exit sites. However, the relationship between the two functions remains unclear. Here we isolated point mutants of cTAGE5 that lost Sec12-binding ability but retained binding to TANGO1. Although expression of the mutant alone could not rescue the defects in collagen VII secretion mediated by cTAGE5 knockdown, coexpression with Sar1, but not with the GTPase-deficient mutant, recovered secretion. The expression of Sar1 alone failed to rescue collagen secretion in cTAGE5-depleted cells. Taken together, these results suggest that two functionally irreplaceable and molecularly separable modules in cTAGE5 are both required for collagen VII export from the ER. The recruitment of Sec12 by cTAGE5 contributes to efficient activation of Sar1 in the vicinity of ER exit sites. In addition, the GTPase cycle of Sar1 appears to be responsible for collagen VII exit from the ER.
© 2016 Tanabe et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

Similar articles
-
Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export.J Cell Biol. 2014 Sep 15;206(6):751-62. doi: 10.1083/jcb.201312062. Epub 2014 Sep 8. J Cell Biol. 2014. PMID: 25202031 Free PMC article.
-
TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers.Proc Natl Acad Sci U S A. 2018 Dec 26;115(52):E12255-E12264. doi: 10.1073/pnas.1814810115. Epub 2018 Dec 13. Proc Natl Acad Sci U S A. 2018. PMID: 30545919 Free PMC article.
-
cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites.Mol Biol Cell. 2011 Jul 1;22(13):2301-8. doi: 10.1091/mbc.E11-02-0143. Epub 2011 Apr 27. Mol Biol Cell. 2011. PMID: 21525241 Free PMC article.
-
Protein export at the ER: loading big collagens into COPII carriers.EMBO J. 2011 Aug 31;30(17):3475-80. doi: 10.1038/emboj.2011.255. EMBO J. 2011. PMID: 21878990 Free PMC article. Review.
-
Mechanisms for exporting large-sized cargoes from the endoplasmic reticulum.Cell Mol Life Sci. 2015 Oct;72(19):3709-20. doi: 10.1007/s00018-015-1952-9. Epub 2015 Jun 17. Cell Mol Life Sci. 2015. PMID: 26082182 Free PMC article. Review.
Cited by
-
TANGO1 Dances to Export of Procollagen from the Endoplasmic Reticulum.Fibrosis (Hong Kong). 2023 Dec;1(2):10008. doi: 10.35534/fibrosis.2023.10008. Epub 2023 Dec 21. Fibrosis (Hong Kong). 2023. PMID: 38650832 Free PMC article.
-
Fatty-acid binding protein 5 modulates the SAR1 GTPase cycle and enhances budding of large COPII cargoes.Mol Biol Cell. 2019 Feb 1;30(3):387-399. doi: 10.1091/mbc.E18-09-0548. Epub 2018 Nov 28. Mol Biol Cell. 2019. PMID: 30485159 Free PMC article.
-
Dual function for Tango1 in secretion of bulky cargo and in ER-Golgi morphology.Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):E10389-E10398. doi: 10.1073/pnas.1711408114. Epub 2017 Nov 14. Proc Natl Acad Sci U S A. 2017. PMID: 29138315 Free PMC article.
-
Coordinated reprogramming of renal cancer transcriptome, metabolome and secretome associates with immune tumor infiltration.Cancer Cell Int. 2023 Jan 5;23(1):2. doi: 10.1186/s12935-022-02845-y. Cancer Cell Int. 2023. PMID: 36604669 Free PMC article.
-
Regulation of the Sar1 GTPase Cycle Is Necessary for Large Cargo Secretion from the Endoplasmic Reticulum.Front Cell Dev Biol. 2017 Aug 23;5:75. doi: 10.3389/fcell.2017.00075. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28879181 Free PMC article. Review.
References
-
- Ishikawa Y, Boudko S, Bachinger HP. Ziploc-ing the structure: triple helix formation is coordinated by rough endoplasmic reticulum resident PPIases. Biochim Biophys Acta. 2015;1850:1983–1993. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

