Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis
- PMID: 27169879
- PMCID: PMC4939511
- DOI: 10.3904/kjim.2016.098
Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis
Abstract
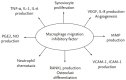
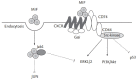
Macrophage migration inhibitory factor (MIF) is originally identified in the culture medium of activated T lymphocytes as a soluble factor that inhibits the random migration of macrophages. MIF is now recognized as a multipotent cytokine involved in the regulation of immune and inf lammatory responses. In rheumatoid arthritis (RA), MIF promotes inf lammatory responses by inducing proinflammatory cytokines and tissue-degrading molecules, promoting the proliferation and survival of synovial fibroblasts, stimulating neutrophil chemotaxis, and regulating angiogenesis and osteoclast differentiation. Expression of MIF in synovial tissue and synovial fluid levels of MIF are elevated in RA patients. Specifically, MIF levels correlate with RA disease activity and high levels are associated with bone erosion. In animal models of RA, the genetic and therapeutic inhibition of MIF has been shown to control inflammation and bone destruction. Based on the role of MIF in RA pathogenesis, small molecular inhibitors targeting it or its receptor pathways could provide a new therapeutic option for RA patients.
Keywords: Arthritis, rheumatoid; Inflammation; Macrophage migration-inhibitory factors; Small molecular inhibitor.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Macrophage migration inhibitory factor: a mediator of matrix metalloproteinase-2 production in rheumatoid arthritis.Arthritis Res Ther. 2006;8(4):R132. doi: 10.1186/ar2021. Arthritis Res Ther. 2006. PMID: 16872482 Free PMC article.
-
Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus.Expert Opin Ther Targets. 2019 Sep;23(9):733-744. doi: 10.1080/14728222.2019.1656718. Epub 2019 Aug 20. Expert Opin Ther Targets. 2019. PMID: 31414920 Free PMC article. Review.
-
The role of macrophage migration inhibitory factor in the inflammatory immune response and rheumatoid arthritis.Wien Med Wochenschr. 2006 Jan;156(1-2):11-8. doi: 10.1007/s10354-005-0243-8. Wien Med Wochenschr. 2006. PMID: 16465610 Review.
-
Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis.J Rheumatol. 2007 May;34(5):927-36. Epub 2007 Apr 1. J Rheumatol. 2007. PMID: 17407222
-
Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids.Arthritis Rheum. 1999 Aug;42(8):1601-8. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. Arthritis Rheum. 1999. PMID: 10446857
Cited by
-
MIF and CD74 as Emerging Biomarkers for Immune Checkpoint Blockade Therapy.Cancers (Basel). 2024 May 4;16(9):1773. doi: 10.3390/cancers16091773. Cancers (Basel). 2024. PMID: 38730725 Free PMC article. Review.
-
Targeting matrix metalloproteases: A promising strategy for herbal medicines to treat rheumatoid arthritis.Front Immunol. 2022 Nov 9;13:1046810. doi: 10.3389/fimmu.2022.1046810. eCollection 2022. Front Immunol. 2022. PMID: 36439173 Free PMC article. Review.
-
Canonical (CD74/CD44) and Non-Canonical (CXCR2, 4 and 7) MIF Receptors Are Differentially Expressed in Rheumatoid Arthritis Patients Evaluated by DAS28-ESR.J Clin Med. 2021 Dec 27;11(1):120. doi: 10.3390/jcm11010120. J Clin Med. 2021. PMID: 35011861 Free PMC article.
-
The Novel Role of MIF in the Secretion of IL-25, IL-31, and IL-33 from PBMC of Patients with Rheumatoid Arthritis.Molecules. 2021 Aug 17;26(16):4968. doi: 10.3390/molecules26164968. Molecules. 2021. PMID: 34443554 Free PMC article.
-
Efficacy of Moxa-burning heat stimulating Zusanli (ST36) and Shenshu (BL23) on expressions of macrophage migration inhibitory factor and macrophage apoptosis in rabbits with adjuvant-induced arthritis.J Tradit Chin Med. 2022 Dec;42(6):980-987. doi: 10.19852/j.cnki.jtcm.20220817.001. J Tradit Chin Med. 2022. PMID: 36378057 Free PMC article.
References
-
- Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford) 2009;48:11–22. - PubMed
-
- Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88. - PubMed
-
- Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
