Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs
- PMID: 27169750
- PMCID: PMC4867683
- DOI: 10.1038/srep24709
Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs
Abstract
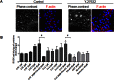
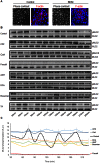
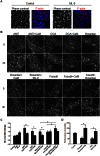
Intrahepatic cholestasis represents a frequent manifestation of drug-induced liver injury; however, the mechanisms underlying such injuries are poorly understood. In this study of human HepaRG and primary hepatocytes, we found that bile canaliculi (BC) underwent spontaneous contractions, which are essential for bile acid (BA) efflux and require alternations in myosin light chain (MLC2) phosphorylation/dephosphorylation. Short exposure to 6 cholestatic compounds revealed that BC constriction and dilation were associated with disruptions in the ROCK/MLCK/myosin pathway. At the studied concentrations, cyclosporine A and chlorpromazine induced early ROCK activity, resulting in permanent MLC2 phosphorylation and BC constriction. However, fasudil reduced ROCK activity and caused rapid, substantial and permanent MLC2 dephosphorylation, leading to BC dilation. The remaining compounds (1-naphthyl isothiocyanate, deoxycholic acid and bosentan) caused BC dilation without modulating ROCK activity, although they were associated with a steady decrease in MLC2 phosphorylation via MLCK. These changes were associated with a common loss of BC contractions and failure of BA clearance. These results provide the first demonstration that cholestatic drugs alter BC dynamics by targeting the ROCK/MLCK pathway; in addition, they highlight new insights into the mechanisms underlying bile flow failure and can be used to identify new predictive biomarkers of drug-induced cholestasis.
Conflict of interest statement
Ruoya Li and Christiane Guguen-Guillouzo are employed by Biopredic International, St Grégoire, France.
Figures




Similar articles
-
Early Alterations of Bile Canaliculi Dynamics and the Rho Kinase/Myosin Light Chain Kinase Pathway Are Characteristics of Drug-Induced Intrahepatic Cholestasis.Drug Metab Dispos. 2016 Nov;44(11):1780-1793. doi: 10.1124/dmd.116.071373. Epub 2016 Aug 18. Drug Metab Dispos. 2016. PMID: 27538918
-
From the Cover: MechanisticInsights in Cytotoxic and Cholestatic Potential of the Endothelial Receptor Antagonists Using HepaRG Cells.Toxicol Sci. 2017 Jun 1;157(2):451-464. doi: 10.1093/toxsci/kfx062. Toxicol Sci. 2017. PMID: 28369585
-
Mycophenolic acid mediated disruption of the intestinal epithelial tight junctions.Exp Cell Res. 2014 Apr 1;322(2):277-89. doi: 10.1016/j.yexcr.2014.01.021. Epub 2014 Feb 7. Exp Cell Res. 2014. PMID: 24509232
-
Regulation of myosin II during cytokinesis in higher eukaryotes.Trends Cell Biol. 2005 Jul;15(7):371-7. doi: 10.1016/j.tcb.2005.05.004. Trends Cell Biol. 2005. PMID: 15935670 Review.
-
In search of a gene for hereditary cholestasis.Biochem Mol Med. 1996 Dec;59(2):98-103. doi: 10.1006/bmme.1996.0073. Biochem Mol Med. 1996. PMID: 8986630 Review. No abstract available.
Cited by
-
Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice.Front Pharmacol. 2018 Oct 4;9:1021. doi: 10.3389/fphar.2018.01021. eCollection 2018. Front Pharmacol. 2018. PMID: 30337870 Free PMC article.
-
In vitro prediction of drug-induced cholestatic liver injury: a challenge for the toxicologist.Arch Toxicol. 2018 May;92(5):1909-1912. doi: 10.1007/s00204-018-2201-4. Epub 2018 Mar 24. Arch Toxicol. 2018. PMID: 29574564 Free PMC article. No abstract available.
-
Cholestasis Differentially Affects Liver Connexins.Int J Mol Sci. 2020 Sep 7;21(18):6534. doi: 10.3390/ijms21186534. Int J Mol Sci. 2020. PMID: 32906817 Free PMC article.
-
Blood-Bile Barrier: Morphology, Regulation, and Pathophysiology.Gene Expr. 2019 Apr 18;19(2):69-87. doi: 10.3727/105221619X15469715711907. Epub 2019 Jan 15. Gene Expr. 2019. PMID: 30646969 Free PMC article. Review.
-
A Predictive 3D Multi-Scale Model of Biliary Fluid Dynamics in the Liver Lobule.Cell Syst. 2017 Mar 22;4(3):277-290.e9. doi: 10.1016/j.cels.2017.02.008. Epub 2017 Mar 18. Cell Syst. 2017. PMID: 28330614 Free PMC article.
References
-
- Bjornsson E. S. & Jonasson J. G. Drug-induced cholestasis. Clin Liver Dis 17, 191–209 (2013). - PubMed
-
- Morgan R. E. et al. A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci 136, 216–241 (2013). - PubMed
-
- Stieger B. Role of the bile salt export pump, BSEP, in acquired forms of cholestasis. Drug Metab Rev 42, 437–445 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

