Functional and structural properties of ion channels at the nerve terminal depends on compact myelin
- PMID: 27168396
- PMCID: PMC5043032
- DOI: 10.1113/JP272205
Functional and structural properties of ion channels at the nerve terminal depends on compact myelin
Abstract
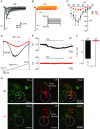
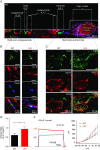
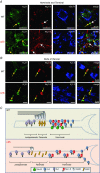
Key points: In the present study, we document the role of compact myelin in regulating the structural and functional properties of ion channels at the nerve terminals, using electrophysiology, dynamic Na(+) imaging and immunohistochemistry. The subcellular segregation of Na(+) channel expression and intracellular Na(+) dynamics at the heminode and terminal was lost in the dysmyelinated axon from Long-Evans shaker rats, which lack compact myelin. In Long-Evans shaker rats, loss of the Nav β4 subunit specifically at the heminode reduced resurgent and persistent Na(+) currents, whereas K(+) channel expression and currents were increased. The results of the present study suggest that there is a specific role for compact myelin in dictating protein expression and function at the axon heminode and in regulating excitability of the nerve terminal.
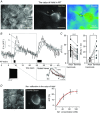
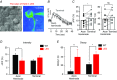
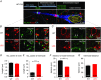
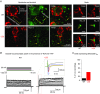
Abstract: Axon myelination increases the conduction velocity and precision of action potential propagation. Although the negative effects of demyelination are generally attributed to conduction failure, accumulating evidence suggests that myelination also regulates the structural properties and molecular composition of the axonal membrane. In the present study, we investigated how myelination affects ion channel expression and function, particularly at the last axon heminode before the nerve terminal, which regulates the presynaptic excitability of the nerve terminal. We compared the structure and physiology of normal axons and those of the Long-Evans shaker (LES) rat, which lacks compact myelin. The normal segregation of Na(+) channel expression and dynamics at the heminode and terminal was lost in the LES rat. Specifically, NaV -α subunits were dispersed and NaV β4 subunit was absent, whereas the density of K(+) channels was increased at the heminode. Correspondingly, resurgent and persistent Na(+) currents were reduced and K(+) current was increased. Taken together, these data suggest a specific role for compact myelin in the orchestration of ion channel expression and function at the axon heminode and in regulating excitability of the nerve terminal.
Keywords: Calyx of held; Kv channels; Myelin; Nav channels; Presynaptic terminal.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
Similar articles
-
Activity-dependent formation and location of voltage-gated sodium channel clusters at a CNS nerve terminal during postnatal development.J Neurophysiol. 2017 Feb 1;117(2):582-593. doi: 10.1152/jn.00617.2016. Epub 2016 Nov 9. J Neurophysiol. 2017. PMID: 27832602 Free PMC article.
-
Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse.J Neurosci. 2005 Apr 6;25(14):3724-38. doi: 10.1523/JNEUROSCI.3983-04.2005. J Neurosci. 2005. PMID: 15814803 Free PMC article.
-
Dysmyelination of auditory afferent axons increases the jitter of action potential timing during high-frequency firing.J Neurosci. 2013 May 29;33(22):9402-7. doi: 10.1523/JNEUROSCI.3389-12.2013. J Neurosci. 2013. PMID: 23719808 Free PMC article.
-
Molecular dissection of the myelinated axon.Ann Neurol. 1993 Feb;33(2):121-36. doi: 10.1002/ana.410330202. Ann Neurol. 1993. PMID: 7679565 Review.
-
Ion channel redistribution and function during development of the myelinated axon.J Neurobiol. 1998 Oct;37(1):80-96. J Neurobiol. 1998. PMID: 9777734 Review.
Cited by
-
Using ephaptic coupling to estimate the synaptic cleft resistivity of the calyx of Held synapse.PLoS Comput Biol. 2021 Oct 26;17(10):e1009527. doi: 10.1371/journal.pcbi.1009527. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34699519 Free PMC article.
-
Diverse Intrinsic Properties Shape Functional Phenotype of Low-Frequency Neurons in the Auditory Brainstem.Front Cell Neurosci. 2018 Jun 26;12:175. doi: 10.3389/fncel.2018.00175. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29997479 Free PMC article.
-
Identification of Persistent and Resurgent Sodium Currents in Spiral Ganglion Neurons Cultured from the Mouse Cochlea.eNeuro. 2017 Nov 14;4(6):ENEURO.0303-17.2017. doi: 10.1523/ENEURO.0303-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29138759 Free PMC article.
-
Restoration of spinal cord injury: From endogenous repairing process to cellular therapy.Front Cell Neurosci. 2022 Nov 29;16:1077441. doi: 10.3389/fncel.2022.1077441. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36523818 Free PMC article. Review.
-
Presynaptic Mitochondria Volume and Abundance Increase during Development of a High-Fidelity Synapse.J Neurosci. 2019 Oct 9;39(41):7994-8012. doi: 10.1523/JNEUROSCI.0363-19.2019. Epub 2019 Aug 27. J Neurosci. 2019. PMID: 31455662 Free PMC article.
References
-
- Alle H & Geiger JR (2006). Combined analog and action potential coding in hippocampal mossy fibers. Science 311, 1290–1293. - PubMed
-
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS & Matthews G (2001). Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 30, 91–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

