Parabrachial CGRP Neurons Control Meal Termination
- PMID: 27166945
- PMCID: PMC4867080
- DOI: 10.1016/j.cmet.2016.04.006
Parabrachial CGRP Neurons Control Meal Termination
Abstract
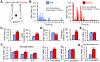
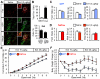
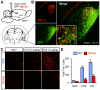
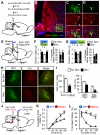
The lateral parabrachial nucleus is a conduit for visceral signals that cause anorexia. We previously identified a subset of neurons located in the external lateral parabrachial nucleus (PBel) that express calcitonin gene-related peptide (CGRP) and inhibit feeding when activated by illness mimetics. We report here that in otherwise normal mice, functional inactivation of CGRP neurons markedly increases meal size, with meal frequency being reduced in a compensatory manner, and renders mice insensitive to the anorexic effects of meal-related satiety peptides. Furthermore, CGRP neurons are directly innervated by orexigenic hypothalamic AgRP neurons, and photostimulation of AgRP fibers supplying the PBel delays satiation by inhibiting CGRP neurons, thereby contributing to AgRP-driven hyperphagia. By establishing a role for CGRP neurons in the control of meal termination and as a downstream mediator of feeding elicited by AgRP neurons, these findings identify a node in which hunger and satiety circuits interact to control feeding behavior.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons.J Neurosci. 2017 Sep 6;37(36):8678-8687. doi: 10.1523/JNEUROSCI.0798-17.2017. Epub 2017 Aug 7. J Neurosci. 2017. PMID: 28821663 Free PMC article.
-
Neurochemical properties of the synapses between the parabrachial nucleus-derived CGRP-positive axonal terminals and the GABAergic neurons in the lateral capsular division of central nucleus of amygdala.Mol Neurobiol. 2015 Feb;51(1):105-18. doi: 10.1007/s12035-014-8713-x. Epub 2014 May 4. Mol Neurobiol. 2015. PMID: 24794145
-
A Parabrachial-to-Amygdala Circuit That Determines Hemispheric Lateralization of Somatosensory Processing.Biol Psychiatry. 2023 Feb 15;93(4):370-381. doi: 10.1016/j.biopsych.2022.09.010. Epub 2022 Sep 16. Biol Psychiatry. 2023. PMID: 36473754 Free PMC article.
-
GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism.Eur J Pharmacol. 2011 Jun 11;660(1):21-7. doi: 10.1016/j.ejphar.2010.10.110. Epub 2011 Jan 3. Eur J Pharmacol. 2011. PMID: 21211531 Free PMC article. Review.
-
Neuroendocrine control of food intake.Nutr Metab Cardiovasc Dis. 2008 Feb;18(2):158-68. doi: 10.1016/j.numecd.2007.06.004. Epub 2007 Dec 3. Nutr Metab Cardiovasc Dis. 2008. PMID: 18061414 Review.
Cited by
-
Neural Circuit Mechanism Underlying the Feeding Controlled by Insula-Central Amygdala Pathway.iScience. 2020 Apr 24;23(4):101033. doi: 10.1016/j.isci.2020.101033. Epub 2020 Apr 5. iScience. 2020. PMID: 32311583 Free PMC article.
-
Danger and distress: Parabrachial-extended amygdala circuits.Neuropharmacology. 2021 Oct 15;198:108757. doi: 10.1016/j.neuropharm.2021.108757. Epub 2021 Aug 27. Neuropharmacology. 2021. PMID: 34461068 Free PMC article. Review.
-
Fast-acting neurons that suppress appetite.Nat Neurosci. 2016 Dec 27;20(1):2-4. doi: 10.1038/nn.4456. Nat Neurosci. 2016. PMID: 28025984 No abstract available.
-
Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors.Neuron. 2017 Mar 22;93(6):1464-1479.e5. doi: 10.1016/j.neuron.2017.02.034. Neuron. 2017. PMID: 28334609 Free PMC article.
-
Feeding signals inhibit fluid-satiation signals in the mouse lateral parabrachial nucleus to increase intake of highly palatable, caloric solutions.J Neurochem. 2023 Dec;167(5):648-667. doi: 10.1111/jnc.15991. Epub 2023 Oct 19. J Neurochem. 2023. PMID: 37855271 Free PMC article.
References
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

