Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk
- PMID: 27166942
- PMCID: PMC4864949
- DOI: 10.1016/j.cmet.2016.04.011
Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk
Abstract
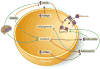
Metabolism research has made tremendous progress over the last several decades in establishing the adipocyte as a central rheostat in the regulation of systemic nutrient and energy homeostasis. Operating at multiple levels of control, the adipocyte communicates with organ systems to adjust gene expression, glucoregulatory hormone exocytosis, enzymatic reactions, and nutrient flux to equilibrate the metabolic demands of a positive or negative energy balance. The identification of these mechanisms has great potential to identify novel targets for the treatment of diabetes and related metabolic disorders. Herein, we review the central role of the adipocyte in the maintenance of metabolic homeostasis, highlighting three critical mediators: adiponectin, leptin, and fatty acids.
Keywords: adipokines; adiponectin; leptin; lipid signaling.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

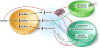
Similar articles
-
Adipose tissue as an endocrine organ.Obesity (Silver Spring). 2006 Aug;14 Suppl 5:242S-249S. doi: 10.1038/oby.2006.317. Obesity (Silver Spring). 2006. PMID: 17021375 Review.
-
Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication.J Lipid Res. 2019 Oct;60(10):1648-1684. doi: 10.1194/jlr.R094060. Epub 2019 Jun 17. J Lipid Res. 2019. PMID: 31209153 Free PMC article. Review.
-
Dichotomous roles of leptin and adiponectin as enforcers against lipotoxicity during feast and famine.Mol Biol Cell. 2013 Oct;24(19):3011-5. doi: 10.1091/mbc.E12-10-0774. Mol Biol Cell. 2013. PMID: 24072813 Free PMC article.
-
Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue.Circ Res. 2005 May 27;96(10):1042-52. doi: 10.1161/01.RES.0000165803.47776.38. Circ Res. 2005. PMID: 15920027 Review.
-
Adipokines regulate systemic insulin sensitivity in accordance to existing energy reserves.Med Hypotheses. 2007;69(1):161-5. doi: 10.1016/j.mehy.2006.10.053. Epub 2007 Jan 8. Med Hypotheses. 2007. PMID: 17208384
Cited by
-
Erythropoietin stimulation of human adipose tissue for therapeutic refilling releases protective cytokines.J Tissue Eng. 2016 Sep 27;7:2041731416671278. doi: 10.1177/2041731416671278. eCollection 2016 Jan-Dec. J Tissue Eng. 2016. PMID: 27738510 Free PMC article.
-
Modern Management of Cardiometabolic Continuum: From Overweight/Obesity to Prediabetes/Type 2 Diabetes Mellitus. Recommendations from the Eastern and Southern Europe Diabetes and Obesity Expert Group.Diabetes Ther. 2024 Sep;15(9):1865-1892. doi: 10.1007/s13300-024-01615-5. Epub 2024 Jul 11. Diabetes Ther. 2024. PMID: 38990471 Free PMC article. Review.
-
Modulation of Leptin and Leptin Receptor Expression in Mice Acutely Infected with Neospora caninum.Pathogens. 2020 Jul 17;9(7):587. doi: 10.3390/pathogens9070587. Pathogens. 2020. PMID: 32709166 Free PMC article.
-
Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis.Adipocyte. 2020 Dec;9(1):302-312. doi: 10.1080/21623945.2020.1778826. Adipocyte. 2020. PMID: 32580621 Free PMC article.
-
Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis.Int J Mol Sci. 2022 Oct 14;23(20):12295. doi: 10.3390/ijms232012295. Int J Mol Sci. 2022. PMID: 36293149 Free PMC article.
References
-
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. - PubMed
-
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochemical and biophysical research communications. 2009;382:51–56. - PubMed
-
- Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

