Steric Effects Induce Geometric Remodeling of Actin Bundles in Filopodia
- PMID: 27166814
- PMCID: PMC4939473
- DOI: 10.1016/j.bpj.2016.03.013
Steric Effects Induce Geometric Remodeling of Actin Bundles in Filopodia
Abstract
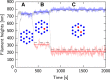
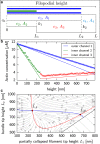
Filopodia are ubiquitous fingerlike protrusions, spawned by many eukaryotic cells, to probe and interact with their environments. Polymerization dynamics of actin filaments, comprising the structural core of filopodia, largely determine their instantaneous lengths and overall lifetimes. The polymerization reactions at the filopodial tip require transport of G-actin, which enter the filopodial tube from the filopodial base and diffuse toward the filament barbed ends near the tip. Actin filaments are mechanically coupled into a tight bundle by cross-linker proteins. Interestingly, many of these proteins are relatively short, restricting the free diffusion of cytosolic G-actin throughout the bundle and, in particular, its penetration into the bundle core. To investigate the effect of steric restrictions on G-actin diffusion by the porous structure of filopodial actin filament bundle, we used a particle-based stochastic simulation approach. We discovered that excluded volume interactions result in partial and then full collapse of central filaments in the bundle, leading to a hollowed-out structure. The latter may further collapse radially due to the activity of cross-linking proteins, hence producing conical-shaped filament bundles. Interestingly, electron microscopy experiments on mature filopodia indeed frequently reveal actin bundles that are narrow at the tip and wider at the base. Overall, our work demonstrates that excluded volume effects in the context of reaction-diffusion processes in porous networks may lead to unexpected geometric growth patterns and complicated, history-dependent dynamics of intermediate metastable configurations.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Helical buckling of actin inside filopodia generates traction.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):136-41. doi: 10.1073/pnas.1411761112. Epub 2014 Dec 22. Proc Natl Acad Sci U S A. 2015. PMID: 25535347 Free PMC article.
-
Molecular noise of capping protein binding induces macroscopic instability in filopodial dynamics.Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11570-5. doi: 10.1073/pnas.0812746106. Epub 2009 Jun 25. Proc Natl Acad Sci U S A. 2009. PMID: 19556544 Free PMC article.
-
Role of the actin bundling protein fascin in growth cone morphogenesis: localization in filopodia and lamellipodia.Cell Motil Cytoskeleton. 2001 Feb;48(2):109-20. doi: 10.1002/1097-0169(200102)48:2<109::AID-CM1002>3.0.CO;2-G. Cell Motil Cytoskeleton. 2001. PMID: 11169763
-
An updated look at actin dynamics in filopodia.Cytoskeleton (Hoboken). 2015 Feb;72(2):71-9. doi: 10.1002/cm.21216. Cytoskeleton (Hoboken). 2015. PMID: 25786787 Review.
-
Regulation of actin assembly associated with protrusion and adhesion in cell migration.Physiol Rev. 2008 Apr;88(2):489-513. doi: 10.1152/physrev.00021.2007. Physiol Rev. 2008. PMID: 18391171 Review.
Cited by
-
Crowding tunes the organization and mechanics of actin bundles formed by crosslinking proteins.FEBS Lett. 2021 Jan;595(1):26-40. doi: 10.1002/1873-3468.13949. Epub 2020 Oct 21. FEBS Lett. 2021. PMID: 33020904 Free PMC article.
-
Actin Bundle Nanomechanics and Organization Are Modulated by Macromolecular Crowding and Electrostatic Interactions.Front Mol Biosci. 2021 Nov 26;8:760950. doi: 10.3389/fmolb.2021.760950. eCollection 2021. Front Mol Biosci. 2021. PMID: 34901154 Free PMC article.
-
Polymerisation force of a rigid filament bundle: diffusive interaction leads to sublinear force-number scaling.Sci Rep. 2018 Feb 6;8(1):2526. doi: 10.1038/s41598-018-20259-7. Sci Rep. 2018. PMID: 29410507 Free PMC article.
-
Multi-resolution dimer models in heat baths with short-range and long-range interactions.Interface Focus. 2019 Jun 6;9(3):20180070. doi: 10.1098/rsfs.2018.0070. Epub 2019 Apr 19. Interface Focus. 2019. PMID: 31065341 Free PMC article.
-
Testing the limits of gradient sensing.PLoS Comput Biol. 2017 Feb 16;13(2):e1005386. doi: 10.1371/journal.pcbi.1005386. eCollection 2017 Feb. PLoS Comput Biol. 2017. PMID: 28207738 Free PMC article.
References
-
- Mattila P.K., Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008;9:446–454. - PubMed
-
- Small J., Rottner K. Elementary cellular processes driven by actin assembly: lamellipodia and filopodia. In: Carlier M.-F., editor. Actin-based Motility. Springer; New York: 2010. pp. 3–33.
-
- Han Y.-H., Chung C.Y., Firtel R.A. Requirement of a vasodilator-stimulated phosphoprotein family member for cell adhesion, the formation of filopodia, and chemotaxis in Dictyostelium. J. Biol. Chem. 2002;277:49877–49887. - PubMed
-
- Dent E.W., Gertler F.B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. - PubMed
-
- Maletic-Savatic M. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

