Genetic Diversity as Consequence of a Microaerobic and Neutrophilic Lifestyle
- PMID: 27166672
- PMCID: PMC4864210
- DOI: 10.1371/journal.ppat.1005626
Genetic Diversity as Consequence of a Microaerobic and Neutrophilic Lifestyle
Abstract
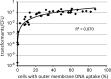
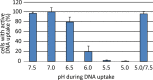
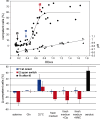
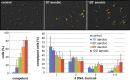
As a neutrophilic bacterium, Helicobacter pylori is growth deficient under extreme acidic conditions. The gastric pathogen is equipped with an acid survival kit, regulating urease activity by a pH-gated urea channel, opening below pH 6.5. After overcoming acid stress, the bacterium's multiplication site is situated at the gastric mucosa with near neutral pH. The pathogen exhibits exceptional genetic variability, mainly due to its capability of natural transformation, termed competence. Using single cell analysis, we show here that competence is highly regulated in H. pylori. DNA uptake complex activity was reversibly shut down below pH 6.5. pH values above 6.5 opened a competence window, in which competence development was triggered by the combination of pH increase and oxidative stress. In contrast, addition of sublethal concentrations of the DNA-damaging agents ciprofloxacin or mitomycin C did not trigger competence development under our conditions. An oxygen-sensitive mutant lacking superoxide dismutase (sodB) displayed a higher competent fraction of cells than the wild type under comparable conditions. In addition, the sodB mutant was dependent on adenine for growth in broth and turned into non-cultivable coccoid forms in its absence, indicating that adenine had radical quenching capacity. Quantification of periplasmically located DNA in competent wild type cells revealed outstanding median imported DNA amounts of around 350 kb per cell within 10 min of import, with maximally a chromosomal equivalent (1.6 Mb) in individual cells, far exceeding previous amounts detected in other Gram-negative bacteria. We conclude that the pathogen's high genetic diversity is a consequence of its enormous DNA uptake capacity, triggered by intrinsic and extrinsic oxidative stress once a neutral pH at the site of chronic host colonization allows competence development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Oxidative stress defense mechanisms to counter iron-promoted DNA damage in Helicobacter pylori.Free Radic Res. 2005 Nov;39(11):1183-91. doi: 10.1080/10715760500194018. Free Radic Res. 2005. PMID: 16298744
-
"Take It or Leave It"-Factors Regulating Competence Development and DNA Uptake in Campylobacter jejuni.Int J Mol Sci. 2021 Sep 21;22(18):10169. doi: 10.3390/ijms221810169. Int J Mol Sci. 2021. PMID: 34576332 Free PMC article.
-
Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells.J Clin Invest. 2000 Aug;106(3):339-47. doi: 10.1172/JCI9351. J Clin Invest. 2000. PMID: 10930437 Free PMC article.
-
Of microbe and man: determinants of Helicobacter pylori-related diseases.FEMS Microbiol Rev. 2006 Jan;30(1):131-56. doi: 10.1111/j.1574-6976.2005.00006.x. FEMS Microbiol Rev. 2006. PMID: 16438683 Review.
-
The gastric biology of Helicobacter pylori.Annu Rev Physiol. 2003;65:349-69. doi: 10.1146/annurev.physiol.65.092101.142156. Epub 2002 May 1. Annu Rev Physiol. 2003. PMID: 12471160 Review.
Cited by
-
ComB proteins expression levels determine Helicobacter pylori competence capacity.Sci Rep. 2017 Jan 27;7:41495. doi: 10.1038/srep41495. Sci Rep. 2017. PMID: 28128333 Free PMC article.
-
Profiling of the Helicobacter pylori redox switch HP1021 regulon using a multi-omics approach.Nat Commun. 2023 Oct 23;14(1):6715. doi: 10.1038/s41467-023-42364-6. Nat Commun. 2023. PMID: 37872172 Free PMC article.
-
HP1021 is a redox switch protein identified in Helicobacter pylori.Nucleic Acids Res. 2021 Jul 9;49(12):6863-6879. doi: 10.1093/nar/gkab440. Nucleic Acids Res. 2021. PMID: 34139017 Free PMC article.
-
The Helicobacter pylori UvrC Nuclease Is Essential for Chromosomal Microimports after Natural Transformation.mBio. 2022 Aug 30;13(4):e0181122. doi: 10.1128/mbio.01811-22. Epub 2022 Jul 25. mBio. 2022. PMID: 35876509 Free PMC article.
-
Cj0683 Is a Competence Protein Essential for Efficient Initialization of DNA Uptake in Campylobacter jejuni.Biomolecules. 2023 Mar 11;13(3):514. doi: 10.3390/biom13030514. Biomolecules. 2023. PMID: 36979449 Free PMC article.
References
-
- Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22(2):283–97. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

