Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site engineering of sphingomyelin synthases
- PMID: 27165857
- PMCID: PMC4918856
- DOI: 10.1194/jlr.M068692
Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site engineering of sphingomyelin synthases
Retraction in
-
ERRATUM.J Lipid Res. 2017 Apr;58(4):821. doi: 10.1194/jlr.M068692ERR. J Lipid Res. 2017. PMID: 28365668 Free PMC article. No abstract available.
Abstract
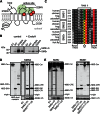
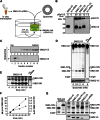
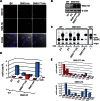
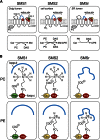
SM is a fundamental component of mammalian cell membranes that contributes to mechanical stability, signaling, and sorting. Its production involves the transfer of phosphocholine from phosphatidylcholine onto ceramide, a reaction catalyzed by SM synthase (SMS) 1 in the Golgi and SMS2 at the plasma membrane. Mammalian cells also synthesize trace amounts of the SM analog ceramide phosphoethanolamine (CPE), but the physiological relevance of CPE production is unclear. Previous work revealed that SMS2 is a bifunctional enzyme producing both SM and CPE, whereas a closely related enzyme, sphingomyelin synthase-related protein (SMSr)/SAMD8, acts as a monofunctional CPE synthase in the endoplasmatic reticulum. Using domain swapping and site-directed mutagenesis on enzymes expressed in defined lipid environments, we here identified structural determinants that mediate head group selectivity of SMS family members. Notably, a single residue adjacent to the catalytic histidine in the third exoplasmic loop profoundly influenced enzyme specificity, with glutamic acid permitting SMS-catalyzed CPE production and aspartic acid confining the enzyme to produce SM. An exchange of exoplasmic residues with SMSr proved sufficient to convert SMS1 into a bulk CPE synthase. This allowed us to establish mammalian cells that produce CPE rather than SM as the principal phosphosphingolipid and provide a model of the molecular interactions that impart catalytic specificity among SMS enzymes.
Keywords: Golgi apparatus; cell-free expression; ceramide phosphoethanolamine; click chemistry; enzyme mechanisms; lipid biochemistry; lipidomics; model membranes; protein engineering.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Switching head group selectivity in mammalian sphingolipid biosynthesis by active-site-engineering of sphingomyelin synthases.J Lipid Res. 2017 May;58(5):962-973. doi: 10.1194/jlr.M076133. Epub 2017 Mar 23. J Lipid Res. 2017. PMID: 28336574 Free PMC article.
-
Sphingomyelin synthase SMS2 displays dual activity as ceramide phosphoethanolamine synthase.J Lipid Res. 2009 Nov;50(11):2270-7. doi: 10.1194/jlr.M900230-JLR200. Epub 2009 May 19. J Lipid Res. 2009. PMID: 19454763 Free PMC article.
-
Functional characterization of enzymes catalyzing ceramide phosphoethanolamine biosynthesis in mice.J Lipid Res. 2015 Apr;56(4):821-35. doi: 10.1194/jlr.M055269. Epub 2015 Feb 9. J Lipid Res. 2015. PMID: 25667419 Free PMC article.
-
Biological functions of sphingomyelins.Prog Lipid Res. 2013 Oct;52(4):424-37. doi: 10.1016/j.plipres.2013.05.001. Epub 2013 May 14. Prog Lipid Res. 2013. PMID: 23684760 Review.
-
Role of ceramide/sphingomyelin (SM) balance regulated through "SM cycle" in cancer.Cell Signal. 2021 Nov;87:110119. doi: 10.1016/j.cellsig.2021.110119. Epub 2021 Aug 19. Cell Signal. 2021. PMID: 34418535 Review.
Cited by
-
Monitoring Changes in the Oligomeric State of a Candidate Endoplasmic Reticulum (ER) Ceramide Sensor by Single-molecule Photobleaching.J Biol Chem. 2016 Nov 18;291(47):24735-24746. doi: 10.1074/jbc.M116.749812. Epub 2016 Oct 10. J Biol Chem. 2016. PMID: 27729449 Free PMC article.
-
Circadian Regulation and Clock-Controlled Mechanisms of Glycerophospholipid Metabolism from Neuronal Cells and Tissues to Fibroblasts.Mol Neurobiol. 2022 Jan;59(1):326-353. doi: 10.1007/s12035-021-02595-4. Epub 2021 Oct 26. Mol Neurobiol. 2022. PMID: 34697790 Review.
-
ER residency of the ceramide phosphoethanolamine synthase SMSr relies on homotypic oligomerization mediated by its SAM domain.Sci Rep. 2017 Jan 25;7:41290. doi: 10.1038/srep41290. Sci Rep. 2017. PMID: 28120887 Free PMC article.
References
-
- Slotte J. P. 2013. Biological functions of sphingomyelins. Prog. Lipid Res. 52: 424–437. - PubMed
-
- Gupta A. K., and Rudney H.. 1991. Plasma membrane sphingomyelin and the regulation of HMG-CoA reductase activity and cholesterol biosynthesis in cell cultures. J. Lipid Res. 32: 125–136. - PubMed
-
- Quiroga R., Trenchi A., González Montoro A., Valdez Taubas J., and Maccioni H. J. F.. 2013. Short transmembrane domains with high-volume exoplasmic halves determine retention of type II membrane proteins in the Golgi complex. J. Cell Sci. 126: 5344–5349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

