Collybolide is a novel biased agonist of κ-opioid receptors with potent antipruritic activity
- PMID: 27162327
- PMCID: PMC4889365
- DOI: 10.1073/pnas.1521825113
Collybolide is a novel biased agonist of κ-opioid receptors with potent antipruritic activity
Abstract
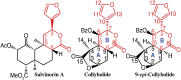
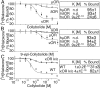
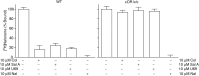
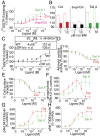
Among the opioid receptors, the κ-opioid receptor (κOR) has been gaining considerable attention as a potential therapeutic target for the treatment of complex CNS disorders including depression, visceral pain, and cocaine addiction. With an interest in discovering novel ligands targeting κOR, we searched natural products for unusual scaffolds and identified collybolide (Colly), a nonnitrogenous sesquiterpene from the mushroom Collybia maculata. This compound has a furyl-δ-lactone core similar to that of Salvinorin A (Sal A), another natural product from the plant Salvia divinorum Characterization of the molecular pharmacological properties reveals that Colly, like Sal A, is a highly potent and selective κOR agonist. However, the two compounds differ in certain signaling and behavioral properties. Colly exhibits 10- to 50-fold higher potency in activating the mitogen-activated protein kinase pathway compared with Sal A. Taken with the fact that the two compounds are equipotent for inhibiting adenylyl cyclase activity, these results suggest that Colly behaves as a biased agonist of κOR. Behavioral studies also support the biased agonistic activity of Colly in that it exhibits ∼10-fold higher potency in blocking non-histamine-mediated itch compared with Sal A, and this difference is not seen in pain attenuation by these two compounds. These results represent a rare example of functional selectivity by two natural products that act on the same receptor. The biased agonistic activity, along with an easily modifiable structure compared with Sal A, makes Colly an ideal candidate for the development of novel therapeutics targeting κOR with reduced side effects.
Keywords: G-protein–coupled receptors; antinociception; dynorphin; natural compounds; salvinorin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Pharmacology and anti-addiction effects of the novel κ opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A.Br J Pharmacol. 2015 Jan;172(2):515-31. doi: 10.1111/bph.12692. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24641310 Free PMC article.
-
Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents.Molecules. 2018 Oct 11;23(10):2602. doi: 10.3390/molecules23102602. Molecules. 2018. PMID: 30314288 Free PMC article.
-
Salvinorin A analogs and other κ-opioid receptor compounds as treatments for cocaine abuse.Adv Pharmacol. 2014;69:481-511. doi: 10.1016/B978-0-12-420118-7.00012-3. Adv Pharmacol. 2014. PMID: 24484985 Free PMC article. Review.
-
Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist.Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11934-9. doi: 10.1073/pnas.182234399. Epub 2002 Aug 21. Proc Natl Acad Sci U S A. 2002. PMID: 12192085 Free PMC article.
-
A review of salvinorin analogs and their kappa-opioid receptor activity.Bioorg Med Chem Lett. 2018 May 15;28(9):1436-1445. doi: 10.1016/j.bmcl.2018.03.029. Epub 2018 Mar 12. Bioorg Med Chem Lett. 2018. PMID: 29615341 Free PMC article. Review.
Cited by
-
Chemical syntheses of the salvinorin chemotype of KOR agonist.Nat Prod Rep. 2020 Nov 18;37(11):1478-1496. doi: 10.1039/d0np00028k. Nat Prod Rep. 2020. PMID: 32808003 Free PMC article. Review.
-
Harnessing cyclotides to design and develop novel peptide GPCR ligands.RSC Chem Biol. 2020 Jul 22;1(4):177-191. doi: 10.1039/d0cb00062k. eCollection 2020 Oct 1. RSC Chem Biol. 2020. PMID: 34458757 Free PMC article. Review.
-
A scoping review of the effects of mushroom and fungus extracts in rodent models of depression and tests of antidepressant activity.Front Pharmacol. 2024 Jun 3;15:1387158. doi: 10.3389/fphar.2024.1387158. eCollection 2024. Front Pharmacol. 2024. PMID: 38887548 Free PMC article.
-
Antinociceptive and Antipruritic Effects of HSK21542, a Peripherally-Restricted Kappa Opioid Receptor Agonist, in Animal Models of Pain and Itch.Front Pharmacol. 2021 Nov 16;12:773204. doi: 10.3389/fphar.2021.773204. eCollection 2021. Front Pharmacol. 2021. PMID: 34867403 Free PMC article.
-
A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists.Front Pharmacol. 2019 Apr 17;10:407. doi: 10.3389/fphar.2019.00407. eCollection 2019. Front Pharmacol. 2019. PMID: 31057409 Free PMC article. Review.
References
-
- Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66(5):285–306. - PubMed
-
- Gavériaux-Ruff C. Opiate-induced analgesia: Contributions from mu, delta and kappa opioid receptors mouse mutants. Curr Pharm Des. 2013;19(42):7373–7381. - PubMed
-
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. - PubMed
-
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242(2):413–420. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

