The contribution of mutant GBA to the development of Parkinson disease in Drosophila
- PMID: 27162249
- PMCID: PMC6390410
- DOI: 10.1093/hmg/ddw129
The contribution of mutant GBA to the development of Parkinson disease in Drosophila
Abstract
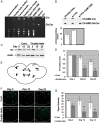
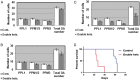
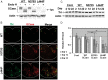
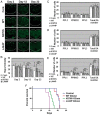
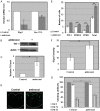
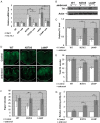
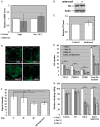
Gaucher disease (GD) results from mutations in the acid β-glucocerebrosidase (GCase) encoding gene, GBA, which leads to accumulation of glucosylceramides. GD patients and carriers of GD mutations have a significantly higher propensity to develop Parkinson disease (PD) in comparison to the non-GD population. In this study, we used the fruit fly Drosophila melanogaster to show that development of PD in carriers of GD mutations results from the presence of mutant GBA alleles. Drosophila has two GBA orthologs (CG31148 and CG31414), each of which has a minos insertion, which creates C-terminal deletion in the encoded GCase. Flies double heterozygous for the endogenous mutant GBA orthologs presented Unfolded Protein Response (UPR) and developed parkinsonian signs, manifested by death of dopaminergic cells, defective locomotion and a shorter life span. We also established transgenic flies carrying the mutant human N370S, L444P and the 84GG variants. UPR activation and development of parkinsonian signs could be recapitulated in flies expressing these three mutant variants.UPR and parkinsonian signs could be partially rescued by growing the double heterozygous flies, or flies expressing the N370S or the L444P human mutant GCase variants, in the presence of the pharmacological chaperone ambroxol, which binds and removes mutant GCase from the endoplasmic reticulum (ER). However flies expressing the 84GG mutant, that does not express mature GCase, did not exhibit rescue by ambroxol. Our results strongly suggest that the presence of a mutant GBA allele in dopaminergic cells leads to ER stress and to their death, and contributes to development of PD.
© The Author 2016. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Unfolded protein response in Gaucher disease: from human to Drosophila.Orphanet J Rare Dis. 2013 Sep 11;8:140. doi: 10.1186/1750-1172-8-140. Orphanet J Rare Dis. 2013. PMID: 24020503 Free PMC article.
-
The effect of mutant GBA1 on accumulation and aggregation of α-synuclein.Hum Mol Genet. 2019 Jun 1;28(11):1768-1781. doi: 10.1093/hmg/ddz005. Hum Mol Genet. 2019. PMID: 30615125
-
Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models.Sci Rep. 2016 Aug 19;6:31380. doi: 10.1038/srep31380. Sci Rep. 2016. PMID: 27539639 Free PMC article.
-
Combined beta-glucosylceramide and ambroxol hydrochloride in patients with Gaucher related Parkinson disease: From clinical observations to drug development.Blood Cells Mol Dis. 2018 Feb;68:117-120. doi: 10.1016/j.bcmd.2016.10.028. Epub 2016 Nov 12. Blood Cells Mol Dis. 2018. PMID: 27866808 Review.
-
Glucocerebrosidase and Parkinson disease: Recent advances.Mol Cell Neurosci. 2015 May;66(Pt A):37-42. doi: 10.1016/j.mcn.2015.03.013. Epub 2015 Mar 20. Mol Cell Neurosci. 2015. PMID: 25802027 Free PMC article. Review.
Cited by
-
Misfolding of Lysosomal α-Galactosidase a in a Fly Model and Its Alleviation by the Pharmacological Chaperone Migalastat.Int J Mol Sci. 2020 Oct 7;21(19):7397. doi: 10.3390/ijms21197397. Int J Mol Sci. 2020. PMID: 33036426 Free PMC article.
-
a-Synuclein and lipids in erythrocytes of Gaucher disease carriers and patients before and after enzyme replacement therapy.PLoS One. 2023 Feb 3;18(2):e0277602. doi: 10.1371/journal.pone.0277602. eCollection 2023. PLoS One. 2023. PMID: 36735655 Free PMC article.
-
Animal models of Parkinson's disease: bridging the gap between disease hallmarks and research questions.Transl Neurodegener. 2023 Jul 19;12(1):36. doi: 10.1186/s40035-023-00368-8. Transl Neurodegener. 2023. PMID: 37468944 Free PMC article. Review.
-
Genetic Evidence for Endolysosomal Dysfunction in Parkinson's Disease: A Critical Overview.Int J Mol Sci. 2023 Mar 28;24(7):6338. doi: 10.3390/ijms24076338. Int J Mol Sci. 2023. PMID: 37047309 Free PMC article. Review.
-
Autophagic dysfunction and gut microbiota dysbiosis cause chronic immune activation in a Drosophila model of Gaucher disease.PLoS Genet. 2023 Dec 21;19(12):e1011063. doi: 10.1371/journal.pgen.1011063. eCollection 2023 Dec. PLoS Genet. 2023. PMID: 38127816 Free PMC article.
References
-
- Beutler E. (1980) Gaucher’s disease. Compr Ther, 6, 65–68. - PubMed
-
- Beutler E. (1999) Gaucher disease. Arch. Intern. Med., 159, 881–882. - PubMed
-
- Brady R.O., Kanfer J.N., Shapiro D. (1965) Metabolism of Glucocerebrosides. Ii. Evidence of an Enzymatic Deficiency in Gaucher's Disease. Biochem. Biophys. Res. Commun., 18, 221–225. - PubMed
-
- Hruska K.S., LaMarca M.E., Scott C.R., Sidransky E. (2008) Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum. Mutat., 29, 567–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

