Enrichment of Scleroderma Vascular Disease-Associated Autoantigens in Endothelial Lineage Cells
- PMID: 27159521
- PMCID: PMC5042822
- DOI: 10.1002/art.39743
Enrichment of Scleroderma Vascular Disease-Associated Autoantigens in Endothelial Lineage Cells
Abstract
Objective: Scleroderma patients with autoantibodies to CENPs and/or interferon-inducible protein 16 (IFI-16) are at increased risk of severe vascular complications. This study was undertaken to determine whether these autoantigens are enriched in cells of the vasculature.
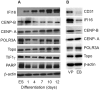
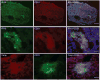
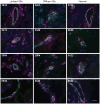
Methods: Successive stages of embryoid bodies (EBs) as well as vascular progenitors were used to evaluate the expression of scleroderma autoantigens IFI-16 and CENP by immunoblotting. CD31 was included to mark early blood vessels. IFI-16 and CD31 expression were defined in paraffin-embedded skin sections from scleroderma patients and from healthy controls. IFI-16 expression was determined by flow cytometric analysis in circulating endothelial cells (CECs) and circulating hematopoietic progenitor cells.
Results: Expression of CENP-A, IFI-16, and CD31 was enriched in EBs on days 10 and 12 of differentiation, and particularly in cultures enriched in vascular progenitors (IFI-16, CD31, and CENPs A and B). This pattern was distinct from that of comparator autoantigens. Immunohistochemical staining of paraffin-embedded skin sections showed enrichment of IFI-16 in CD31-positive vascular endothelial cells in biopsy specimens from scleroderma patients and normal controls. Flow cytometric analysis revealed IFI-16 expression in circulating hematopoietic progenitor cells but minimal expression in CECs.
Conclusion: Our findings indicate that expression of the scleroderma autoantigens IFI-16 and CENPs, which are associated with severe vascular disease, is increased in vascular progenitors and mature endothelial cells. High level, lineage-enriched expression of autoantigens may explain the striking association between clinical phenotypes and the immune targeting of specific autoantigens.
© 2016, American College of Rheumatology.
Figures


Similar articles
-
Recognition of Granzyme B-generated autoantigen fragments in scleroderma patients with ischemic digital loss.Arthritis Rheum. 2002 Jul;46(7):1873-84. doi: 10.1002/art.10407. Arthritis Rheum. 2002. PMID: 12124872
-
Anti-Interferon-Inducible Protein 16 Antibodies Associate With Digital Gangrene in Patients With Scleroderma.Arthritis Rheumatol. 2016 May;68(5):1262-71. doi: 10.1002/art.39558. Arthritis Rheumatol. 2016. PMID: 26714268 Free PMC article.
-
Fine specificity mapping of autoantigens targeted by anti-centromere autoantibodies.J Autoimmun. 2006 Dec;27(4):272-80. doi: 10.1016/j.jaut.2006.10.001. Epub 2007 Jan 8. J Autoimmun. 2006. PMID: 17210244 Free PMC article.
-
Autoantibodies in systemic sclerosis (scleroderma): clues for clinical evaluation, prognosis and pathogenesis.Wien Med Wochenschr. 2008;158(1-2):19-28. doi: 10.1007/s10354-007-0451-5. Wien Med Wochenschr. 2008. PMID: 18286246 Review.
-
Anti-centromere protein A antibodies in systemic sclerosis: Significance and origin.Autoimmun Rev. 2016 Jan;15(1):102-9. doi: 10.1016/j.autrev.2015.10.001. Epub 2015 Oct 9. Autoimmun Rev. 2016. PMID: 26455561 Review.
Cited by
-
Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis.J Clin Invest. 2020 May 1;130(5):2451-2464. doi: 10.1172/JCI131700. J Clin Invest. 2020. PMID: 31990684 Free PMC article. Clinical Trial.
-
Heterozygous Gnaq deficiency enhances Ifi202b/IFI16 and NF-κB activation in endothelial cells and exacerbates lupus nephritis pathology.iScience. 2024 Jun 22;27(8):110350. doi: 10.1016/j.isci.2024.110350. eCollection 2024 Aug 16. iScience. 2024. PMID: 39108722 Free PMC article.
-
Progress in understanding the diagnostic and pathogenic role of autoantibodies associated with systemic sclerosis.Curr Opin Rheumatol. 2016 Nov;28(6):586-94. doi: 10.1097/BOR.0000000000000325. Curr Opin Rheumatol. 2016. PMID: 27387266 Free PMC article. Review.
-
Toward Blood-Based Precision Medicine: Identifying Age-Sex-Specific Vascular Biomarker Quantities on Circulating Vascular Cells.Cell Mol Bioeng. 2023 Jul 6;16(3):189-204. doi: 10.1007/s12195-023-00771-1. eCollection 2023 Jun. Cell Mol Bioeng. 2023. PMID: 37456786 Free PMC article.
-
Integrative analyses of gene expression profile reveal potential crucial roles of mitotic cell cycle and microtubule cytoskeleton in pulmonary artery hypertension.BMC Med Genomics. 2020 Jun 26;13(1):86. doi: 10.1186/s12920-020-00740-x. BMC Med Genomics. 2020. PMID: 32586319 Free PMC article.
References
-
- Greidinger EL, Flaherty KT, White B, Rosen A, Wigley FM, Wise RA. African-American race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest. 1998 Sep;114(3):801–7. - PubMed
-
- Khimdas S, Harding S, Bonner A, Zummer B, Baron M, Pope J, et al. Associations with digital ulcers in a large cohort of systemic sclerosis: results from the Canadian Scleroderma Research Group registry. Arthritis Care Res (Hoboken) 2011 Jan;63(1):142–9. - PubMed
-
- Tiev KP, Diot E, Clerson P, Dupuis-Simeon F, Hachulla E, Hatron PY, et al. Clinical features of scleroderma patients with or without prior or current ischemic digital ulcers: post-hoc analysis of a nationwide multicenter cohort (ItinerAIR-Sclerodermie) J Rheumatol. 2009 Jul;36(7):1470–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

