Natriuretic Peptide Receptor Guanylyl Cyclase-A in Podocytes is Renoprotective but Dispensable for Physiologic Renal Function
- PMID: 27153922
- PMCID: PMC5198264
- DOI: 10.1681/ASN.2015070731
Natriuretic Peptide Receptor Guanylyl Cyclase-A in Podocytes is Renoprotective but Dispensable for Physiologic Renal Function
Abstract
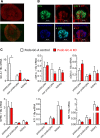
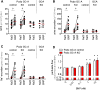
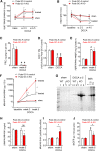
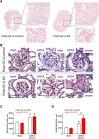
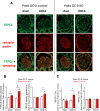
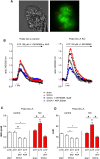
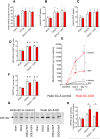
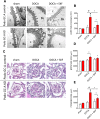
The cardiac natriuretic peptides (NPs), atrial NP and B-type NP, regulate fluid homeostasis and arterial BP through renal actions involving increased GFR and vascular and tubular effects. Guanylyl cyclase-A (GC-A), the transmembrane cGMP-producing receptor shared by these peptides, is expressed in different renal cell types, including podocytes, where its function is unclear. To study the effects of NPs on podocytes, we generated mice with a podocyte-specific knockout of GC-A (Podo-GC-A KO). Despite the marked reduction of GC-A mRNA in GC-A KO podocytes to 1% of the control level, Podo-GC-A KO mice and control littermates did not differ in BP, GFR, or natriuresis under baseline conditions. Moreover, infusion of synthetic NPs similarly increased the GFR and renal perfusion in both genotypes. Administration of the mineralocorticoid deoxycorticosterone-acetate (DOCA) in combination with high salt intake induced arterial hypertension of similar magnitude in Podo-GC-A KO mice and controls. However, only Podo-GC-A KO mice developed massive albuminuria (controls: 35-fold; KO: 5400-fold versus baseline), hypoalbuminemia, reduced GFR, and marked glomerular damage. Furthermore, DOCA treatment led to decreased expression of the slit diaphragm-associated proteins podocin, nephrin, and synaptopodin and to enhanced transient receptor potential canonical 6 (TRPC6) channel expression and ATP-induced calcium influx in podocytes of Podo-GC-A KO mice. Concomitant treatment of Podo-GC-A KO mice with the TRPC channel blocker SKF96365 markedly ameliorated albuminuria and glomerular damage in response to DOCA. In conclusion, the physiologic effects of NPs on GFR and natriuresis do not involve podocytes. However, NP/GC-A/cGMP signaling protects podocyte integrity under pathologic conditions, most likely by suppression of TRPC channels.
Keywords: albuminuria; cardiac natriuretic peptides; podocyte; progression of renal failure.
Copyright © 2016 by the American Society of Nephrology.
Figures
Similar articles
-
Podocyte cell-specific Npr1 is required for blood pressure and renal homeostasis in male and female mice: role of sex-specific differences.Physiol Genomics. 2024 Oct 1;56(10):672-690. doi: 10.1152/physiolgenomics.00137.2023. Epub 2024 Aug 5. Physiol Genomics. 2024. PMID: 39101921 Free PMC article.
-
Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury.J Am Soc Nephrol. 2012 Jul;23(7):1198-209. doi: 10.1681/ASN.2011100985. Epub 2012 May 31. J Am Soc Nephrol. 2012. PMID: 22652704 Free PMC article.
-
Natriuretic peptide receptor guanylyl cyclase-A pathway counteracts glomerular injury evoked by aldosterone through p38 mitogen-activated protein kinase inhibition.Sci Rep. 2017 Apr 21;7:46624. doi: 10.1038/srep46624. Sci Rep. 2017. PMID: 28429785 Free PMC article.
-
Molecular and genetic aspects of guanylyl cyclase natriuretic peptide receptor-A in regulation of blood pressure and renal function.Physiol Genomics. 2018 Nov 1;50(11):913-928. doi: 10.1152/physiolgenomics.00083.2018. Epub 2018 Aug 31. Physiol Genomics. 2018. PMID: 30169131 Free PMC article. Review.
-
Electrophysiological effects of natriuretic peptides in the heart are mediated by multiple receptor subtypes.Prog Biophys Mol Biol. 2016 Jan;120(1-3):37-49. doi: 10.1016/j.pbiomolbio.2015.12.001. Epub 2015 Dec 15. Prog Biophys Mol Biol. 2016. PMID: 26701223 Review.
Cited by
-
β Cell-specific deletion of guanylyl cyclase A, the receptor for atrial natriuretic peptide, accelerates obesity-induced glucose intolerance in mice.Cardiovasc Diabetol. 2018 Jul 17;17(1):103. doi: 10.1186/s12933-018-0747-3. Cardiovasc Diabetol. 2018. PMID: 30016962 Free PMC article.
-
Podocyte cell-specific Npr1 is required for blood pressure and renal homeostasis in male and female mice: role of sex-specific differences.Physiol Genomics. 2024 Oct 1;56(10):672-690. doi: 10.1152/physiolgenomics.00137.2023. Epub 2024 Aug 5. Physiol Genomics. 2024. PMID: 39101921 Free PMC article.
-
In utero exposure to maternal diabetes impairs nephron progenitor differentiation.Am J Physiol Renal Physiol. 2019 Nov 1;317(5):F1318-F1330. doi: 10.1152/ajprenal.00204.2019. Epub 2019 Sep 11. Am J Physiol Renal Physiol. 2019. PMID: 31509011 Free PMC article.
-
Empagliflozin Reduces Renal Hyperfiltration in Response to Uninephrectomy, but Is Not Nephroprotective in UNx/DOCA/Salt Mouse Models.Front Pharmacol. 2021 Dec 21;12:761855. doi: 10.3389/fphar.2021.761855. eCollection 2021. Front Pharmacol. 2021. PMID: 34992532 Free PMC article.
-
Pharmacologic blockade of the natriuretic peptide clearance receptor promotes weight loss and enhances insulin sensitivity in type 2 diabetes.Transl Res. 2023 May;255:140-151. doi: 10.1016/j.trsl.2022.12.005. Epub 2022 Dec 21. Transl Res. 2023. PMID: 36563959 Free PMC article.
References
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 - PubMed
-
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 - PubMed
-
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 - PubMed
-
- Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 - PMC - PubMed
-
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H: A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

