Galectin-1 is essential for the induction of MOG35-55 -based intravenous tolerance in experimental autoimmune encephalomyelitis
- PMID: 27151444
- PMCID: PMC4984530
- DOI: 10.1002/eji.201546212
Galectin-1 is essential for the induction of MOG35-55 -based intravenous tolerance in experimental autoimmune encephalomyelitis
Abstract
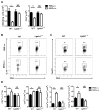
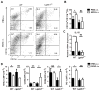
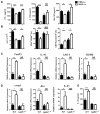
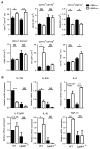
In experimental autoimmune encephalomyelitis (EAE), intravenous (i.v.) injection of the antigen, myelin oligodendrocyte glycoprotein-derived peptide, MOG35-55 , suppresses disease development, a phenomenon called i.v. tolerance. Galectin-1, an endogenous glycan-binding protein, is upregulated during autoimmune neuroinflammation and plays immunoregulatory roles by inducing tolerogenic dendritic cells (DCs) and IL-10 producing regulatory type 1 T (Tr1) cells. To examine the role of galectin-1 in i.v. tolerance, we administered MOG35-55 -i.v. to wild-type (WT) and galectin-1 deficient (Lgals1(-/-) ) mice with ongoing EAE. MOG35-55 suppressed disease in the WT, but not in the Lgals1(-/-) mice. The numbers of Tr1 cells and Treg cells were increased in the CNS and periphery of tolerized WT mice. In contrast, Lgals1(-/-) MOG-i.v. mice had reduced numbers of Tr1 cells and Treg cells in the CNS and periphery, and reduced IL-27, IL-10, and TGF-β1 expression in DCs in the periphery. DCs derived from i.v.-tolerized WT mice suppressed disease when adoptively transferred into mice with ongoing EAE, whereas DCs from Lgals1(-/-) MOG-i.v. mice were not suppressive. These findings demonstrate that galectin-1 is required for i.v. tolerance induction, likely via induction of tolerogenic DCs leading to enhanced development of Tr1 cells, Treg cells, and downregulation of proinflammatory responses.
Keywords: Galectin-1; Tolerance; Tolerogenic DC; Tr1 cell; Treg cell.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures

Similar articles
-
Selective depletion of CD11c+ CD11b+ dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE.Eur J Immunol. 2016 Oct;46(10):2454-2466. doi: 10.1002/eji.201546274. Eur J Immunol. 2016. PMID: 27338697 Free PMC article.
-
A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4+ CD25high FOXP3+ Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition.Front Immunol. 2019 Jan 10;9:3119. doi: 10.3389/fimmu.2018.03119. eCollection 2018. Front Immunol. 2019. PMID: 30687323 Free PMC article.
-
Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis.Exp Neurol. 2015 May;267:254-67. doi: 10.1016/j.expneurol.2014.10.019. Epub 2014 Oct 30. Exp Neurol. 2015. PMID: 25447934
-
Vaccination with autologous dendritic cells: from experimental autoimmune encephalomyelitis to multiple sclerosis.J Neuroimmunol. 2001 Mar 1;114(1-2):1-7. doi: 10.1016/s0165-5728(01)00247-8. J Neuroimmunol. 2001. PMID: 11240009 Review.
-
Tolerogenic Dendritic Cells as a Promising Antigen-Specific Therapy in the Treatment of Multiple Sclerosis and Neuromyelitis Optica From Preclinical to Clinical Trials.Front Immunol. 2018 May 31;9:1169. doi: 10.3389/fimmu.2018.01169. eCollection 2018. Front Immunol. 2018. PMID: 29904379 Free PMC article. Review.
Cited by
-
Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling.Sci Rep. 2018 Feb 27;8(1):3741. doi: 10.1038/s41598-018-22135-w. Sci Rep. 2018. PMID: 29487346 Free PMC article.
-
Induction of Peripheral Tolerance in Ongoing Autoimmune Inflammation Requires Interleukin 27 Signaling in Dendritic Cells.Front Immunol. 2017 Oct 27;8:1392. doi: 10.3389/fimmu.2017.01392. eCollection 2017. Front Immunol. 2017. PMID: 29163476 Free PMC article.
-
Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance.J Clin Invest. 2017 Mar 1;127(3):1046-1060. doi: 10.1172/JCI89927. Epub 2017 Feb 20. J Clin Invest. 2017. PMID: 28218625 Free PMC article.
-
Interferon-γ/Interleukin-27 Axis Induces Programmed Death Ligand 1 Expression in Monocyte-Derived Dendritic Cells and Restores Immune Tolerance in Central Nervous System Autoimmunity.Front Immunol. 2020 Oct 26;11:576752. doi: 10.3389/fimmu.2020.576752. eCollection 2020. Front Immunol. 2020. PMID: 33193372 Free PMC article.
-
Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities.Int J Mol Sci. 2023 Mar 30;24(7):6501. doi: 10.3390/ijms24076501. Int J Mol Sci. 2023. PMID: 37047471 Free PMC article. Review.
References
-
- Weiner HL, Friedman A, Miller A, Khoury SJ, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. - PubMed
-
- Miller A, Zhang ZJ, Sobel RA, al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993;46:73–82. - PubMed
-
- Pitkanen J, Peterson P. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 2003;4:12–21. - PubMed
-
- Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

