Effects of N-Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Ligands
- PMID: 27148755
- PMCID: PMC4885601
- DOI: 10.1021/acs.jmedchem.6b00308
Effects of N-Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Ligands
Abstract
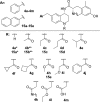
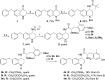
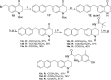
N-Acetylation of the tetrahydroquinoline (THQ) core of a series of μ-opioid receptor (MOR) agonist/δ-opioid receptor (DOR) antagonist ligands increases DOR affinity, resulting in ligands with balanced MOR and DOR affinities. We report a series of N-substituted THQ analogues that incorporate various carbonyl-containing moieties to maintain DOR affinity and define the steric and electronic requirements of the binding pocket across the opioid receptors. 4h produced in vivo antinociception (ip) for 1 h at 10 mg/kg.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


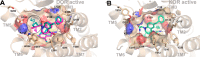
Similar articles
-
Structural Simplification of a Tetrahydroquinoline-Core Peptidomimetic μ-Opioid Receptor (MOR) Agonist/δ-Opioid Receptor (DOR) Antagonist Produces Improved Metabolic Stability.J Med Chem. 2019 Apr 25;62(8):4142-4157. doi: 10.1021/acs.jmedchem.9b00219. Epub 2019 Apr 12. J Med Chem. 2019. PMID: 30924650 Free PMC article.
-
Asymmetric synthesis and in vitro and in vivo activity of tetrahydroquinolines featuring a diverse set of polar substitutions at the 6 position as mixed-efficacy μ opioid receptor/δ opioid receptor ligands.ACS Chem Neurosci. 2015 Aug 19;6(8):1428-35. doi: 10.1021/acschemneuro.5b00100. Epub 2015 May 13. ACS Chem Neurosci. 2015. PMID: 25938166 Free PMC article.
-
Further Optimization and Evaluation of Bioavailable, Mixed-Efficacy μ-Opioid Receptor (MOR) Agonists/δ-Opioid Receptor (DOR) Antagonists: Balancing MOR and DOR Affinities.J Med Chem. 2015 Nov 25;58(22):8952-69. doi: 10.1021/acs.jmedchem.5b01270. Epub 2015 Nov 13. J Med Chem. 2015. PMID: 26524472 Free PMC article.
-
The search for opioid analgesics with limited tolerance liability.Peptides. 2020 Aug;130:170331. doi: 10.1016/j.peptides.2020.170331. Epub 2020 Jun 1. Peptides. 2020. PMID: 32497566 Review.
-
Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics.AAPS J. 2006 Mar 10;8(1):E118-25. doi: 10.1208/aapsj080114. AAPS J. 2006. PMID: 16584118 Free PMC article. Review.
Cited by
-
SAR Matrices Enable Discovery of Mixed Efficacy μ-Opioid Receptor Agonist Peptidomimetics with Simplified Structures through an Aromatic-Amine Pharmacophore.ACS Chem Neurosci. 2021 Jan 6;12(1):216-233. doi: 10.1021/acschemneuro.0c00693. Epub 2020 Dec 21. ACS Chem Neurosci. 2021. PMID: 33346631 Free PMC article.
-
Novel Dimethyltyrosine-Tetrahydroisoquinoline Peptidomimetics with Aromatic Tetrahydroisoquinoline Substitutions Show in Vitro Kappa and Mu Opioid Receptor Agonism.ACS Chem Neurosci. 2019 Aug 21;10(8):3682-3689. doi: 10.1021/acschemneuro.9b00250. Epub 2019 Jul 1. ACS Chem Neurosci. 2019. PMID: 31199621 Free PMC article.
-
Structural Simplification of a Tetrahydroquinoline-Core Peptidomimetic μ-Opioid Receptor (MOR) Agonist/δ-Opioid Receptor (DOR) Antagonist Produces Improved Metabolic Stability.J Med Chem. 2019 Apr 25;62(8):4142-4157. doi: 10.1021/acs.jmedchem.9b00219. Epub 2019 Apr 12. J Med Chem. 2019. PMID: 30924650 Free PMC article.
-
Toward a Universal μ-Agonist Template for Template-Based Alignment Modeling of Opioid Ligands.ACS Omega. 2019 Oct 9;4(17):17457-17476. doi: 10.1021/acsomega.9b02244. eCollection 2019 Oct 22. ACS Omega. 2019. PMID: 31656918 Free PMC article.
-
Novel Molecular Strategies and Targets for Opioid Drug Discovery for the Treatment of Chronic Pain.Yale J Biol Med. 2017 Mar 29;90(1):97-110. eCollection 2017 Mar. Yale J Biol Med. 2017. PMID: 28356897 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

