Immune Responses to Circulating and Vaccine Viral Strains in HIV-Infected and Uninfected Children and Youth Who Received the 2013/2014 Quadrivalent Live-Attenuated Influenza Vaccine
- PMID: 27148262
- PMCID: PMC4831981
- DOI: 10.3389/fimmu.2016.00142
Immune Responses to Circulating and Vaccine Viral Strains in HIV-Infected and Uninfected Children and Youth Who Received the 2013/2014 Quadrivalent Live-Attenuated Influenza Vaccine
Abstract
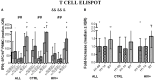
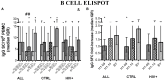
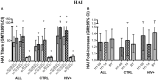
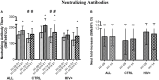
The live-attenuated influenza vaccine (LAIV) has generally been more efficacious than the inactivated vaccine in children. However, LAIV is not recommended for HIV-infected children because of insufficient data. We compared cellular, humoral, and mucosal immune responses to the 2013-2014 LAIV quadrivalent (LAIV4) in HIV-infected and uninfected children 2-25 years of age (yoa). We analyzed the responses to the vaccine H1N1 (H1N1-09), to the circulating H1N1 (H1N1-14), which had significant mutations compared to H1N1-09 and to B Yamagata (BY), which had the highest effectiveness in 2013-2014. Forty-six HIV-infected and 56 uninfected participants with prior influenza immunization had blood and nasal swabs collected before and after LAIV4 for IFNγ T and IgG/IgA memory B-cell responses (ELISPOT), plasma antibodies [hemagglutination inhibition (HAI) and microneutralization (MN)], and mucosal IgA (ELISA). The HIV-infected participants had median CD4+ T cells = 645 cells/μL and plasma HIV RNA = 20 copies/mL. Eighty-four percent were on combination anti-retroviral therapy. Regardless of HIV status, significant increases in T-cell responses were observed against BY, but not against H1N1-09. H1N1-09 T-cell immunity was higher than H1N1-14 both before and after vaccination. LAIV4 significantly increased memory IgG B-cell immunity against H1N1-14 and BY in uninfected, but not in HIV-infected participants. Regardless of HIV status, H1N1-09 memory IgG B-cell immunity was higher than H1N1-14 and lower than BY. There were significant HAI titer increases after vaccination in all groups and against all viruses. However, H1N1-14 MN titers were significantly lower than H1N1-09 before and after vaccination overall and in HIV-uninfected vaccinees. Regardless of HIV status, LAIV4 increased nasal IgA concentrations against all viruses. The fold-increase in H1N1-09 IgA was lower than BY. Overall, participants <9 yoa had decreased BY-specific HAI and nasal IgA responses to LAIV4. In conclusion, HIV-infected and uninfected children and youth had comparable responses to LAIV4. H1N1-09 immune responses were lower than BY and higher than H1N1-14, suggesting that both antigenic mismatches between circulating and vaccine H1N1 and lower immunogenicity of the H1N1 vaccine strain may have contributed to the decreased H1N1 effectiveness of 2013-2014 LAIV4.
Keywords: ELISPOT; HIV infection; LAIV; cell-mediated immunity; children; influenza vaccine; neutralization.
Conflict of interest statement
The authors do not have a commercial or other association that might pose a conflict of interest.
Figures
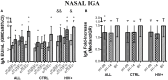
Similar articles
-
Safety, immunogenicity and shedding of LAIV4 in HIV-infected and uninfected children.Vaccine. 2015 Sep 11;33(38):4790-7. doi: 10.1016/j.vaccine.2015.07.082. Epub 2015 Aug 1. Vaccine. 2015. PMID: 26241950
-
Humoral immune response following the inactivated quadrivalent influenza vaccination among HIV-infected and HIV-uninfected adults.Vaccine. 2023 Jul 31;41(34):4978-4985. doi: 10.1016/j.vaccine.2023.05.055. Epub 2023 Jun 30. Vaccine. 2023. PMID: 37394372
-
A study to evaluate the immunogenicity and shedding of live attenuated influenza vaccine strains in children 24-<48 months of age.Vaccine. 2020 Jan 29;38(5):1001-1008. doi: 10.1016/j.vaccine.2019.11.055. Epub 2019 Nov 30. Vaccine. 2020. PMID: 31796225 Clinical Trial.
-
A randomized controlled trial of antibody response to 2019-20 cell-based inactivated and egg-based live attenuated influenza vaccines in children and young adults.Vaccine. 2022 Jan 31;40(5):780-788. doi: 10.1016/j.vaccine.2021.12.034. Epub 2021 Dec 21. Vaccine. 2022. PMID: 34952751 Free PMC article. Clinical Trial.
-
Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review.
Cited by
-
Summary of the NACI systematic review and recommendation on the use of live attenuated influenza vaccine (LAIV) in HIV-infected individuals.Can Commun Dis Rep. 2020 Sep 3;46(9):299-304. doi: 10.14745/ccdr.v46i09a08. eCollection 2020 Sep 3. Can Commun Dis Rep. 2020. PMID: 33104088 Free PMC article.
-
Immunogenicity Measures of Influenza Vaccines: A Study of 1164 Registered Clinical Trials.Vaccines (Basel). 2020 Jun 19;8(2):325. doi: 10.3390/vaccines8020325. Vaccines (Basel). 2020. PMID: 32575440 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza – United States, 1976-2007. MMWR Morb Mortal Wkly Rep (2010) 59:1057–62. - PubMed
-
- Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr (2000) 136:168–75.10.1016/S0022-3476(00)70097-7 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

