New Insights into the Generation of CD4 Memory May Shape Future Vaccine Strategies for Influenza
- PMID: 27148257
- PMCID: PMC4827017
- DOI: 10.3389/fimmu.2016.00136
New Insights into the Generation of CD4 Memory May Shape Future Vaccine Strategies for Influenza
Abstract
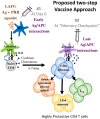
Influenza viral evolution presents a formidable challenge to vaccination due to the virus' ability to rapidly mutate to evade immune responses. Live influenza infections generate large and diverse CD4 effector T cell responses that yield highly protective, long-lasting CD4 T cell memory that can target conserved viral epitopes. We review advances in our understanding of mechanisms involved in generating CD4 T cell responses against the influenza A virus (IAV), focusing on specialized follicular helper (TFH) and CD4 cytotoxic (ThCTL) effector subsets and on CD4 T cell memory. We also discuss two recent findings in context of enhancing vaccine responses. First, helper T cells require priming with APC secreting high levels of IL-6. Second, the transition of IAV-generated effectors to memory depends on IL-2, costimulation and antigen signals, just before effectors reach peak numbers, defined as the "memory checkpoint." The need for these signals during the checkpoint could explain why many current influenza vaccines are poorly effective and elicit poor cellular immunity. We suggest that CD4 memory generation can be enhanced by re-vaccinating at this time. Our best hope lies in a universal vaccine that will not need to be formulated yearly against seasonal antigenically novel influenza strains and will also be protective against a pandemic strain. We suggest a vaccine approach that elicits a powerful T cell response, by initially inducing high levels of APC activation and later providing antigen at the memory checkpoint, may take us a step closer to such a universal influenza vaccine.
Keywords: CD4 T cells; cell-mediated immunity; influenza; late-antigen; memory checkpoint; vaccination.
Figures
Similar articles
-
Pathogen Recognition by CD4 Effectors Drives Key Effector and Most Memory Cell Generation Against Respiratory Virus.Front Immunol. 2018 Mar 26;9:596. doi: 10.3389/fimmu.2018.00596. eCollection 2018. Front Immunol. 2018. PMID: 29632538 Free PMC article. Review.
-
Influenza Vaccine-Induced CD4 Effectors Require Antigen Recognition at an Effector Checkpoint to Generate CD4 Lung Memory and Antibody Production.J Immunol. 2020 Oct 15;205(8):2077-2090. doi: 10.4049/jimmunol.2000597. Epub 2020 Sep 14. J Immunol. 2020. PMID: 32929040 Free PMC article.
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.bioRxiv [Preprint]. 2021 Mar 25:2021.03.24.436901. doi: 10.1101/2021.03.24.436901. bioRxiv. 2021. Update in: J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. PMID: 33791695 Free PMC article. Updated. Preprint.
-
Protein Vaccination Directs the CD4+ T Cell Response toward Shared Protective Epitopes That Can Be Recalled after Influenza Virus Infection.J Virol. 2019 Sep 30;93(20):e00947-19. doi: 10.1128/JVI.00947-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341045 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
Cited by
-
Strong influenza-induced TFH generation requires CD4 effectors to recognize antigen locally and receive signals from continuing infection.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2111064119. doi: 10.1073/pnas.2111064119. Proc Natl Acad Sci U S A. 2022. PMID: 35177472 Free PMC article.
-
Fighting Viral Infections and Virus-Driven Tumors with Cytotoxic CD4+ T Cells.Front Immunol. 2017 Feb 27;8:197. doi: 10.3389/fimmu.2017.00197. eCollection 2017. Front Immunol. 2017. PMID: 28289418 Free PMC article. Review.
-
Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity.Mucosal Immunol. 2022 Apr;15(4):717-729. doi: 10.1038/s41385-022-00497-9. Epub 2022 Mar 8. Mucosal Immunol. 2022. PMID: 35260804 Free PMC article.
-
Pathogen Recognition by CD4 Effectors Drives Key Effector and Most Memory Cell Generation Against Respiratory Virus.Front Immunol. 2018 Mar 26;9:596. doi: 10.3389/fimmu.2018.00596. eCollection 2018. Front Immunol. 2018. PMID: 29632538 Free PMC article. Review.
-
Durable CD4 T-Cell Memory Generation Depends on Persistence of High Levels of Infection at an Effector Checkpoint that Determines Multiple Fates.Cold Spring Harb Perspect Biol. 2021 Nov 1;13(11):a038182. doi: 10.1101/cshperspect.a038182. Cold Spring Harb Perspect Biol. 2021. PMID: 33903157 Free PMC article. Review.
References
-
- CDC. Seasonal Influenza Vaccine Effectiveness, 2005-2015. (2015). Available from: http://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

