Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes
- PMID: 27145372
- PMCID: PMC5058660
- DOI: 10.18632/oncotarget.9065
Vitamin D receptor signaling improves Hutchinson-Gilford progeria syndrome cellular phenotypes
Abstract
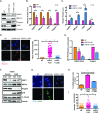
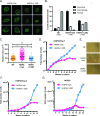
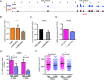
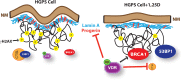
Hutchinson-Gilford Progeria Syndrome (HGPS) is a devastating incurable premature aging disease caused by accumulation of progerin, a toxic lamin A mutant protein. HGPS patient-derived cells exhibit nuclear morphological abnormalities, altered signaling pathways, genomic instability, and premature senescence. Here we uncover new molecular mechanisms contributing to cellular decline in progeria. We demonstrate that HGPS cells reduce expression of vitamin D receptor (VDR) and DNA repair factors BRCA1 and 53BP1 with progerin accumulation, and that reconstituting VDR signaling via 1α,25-dihydroxyvitamin D3 (1,25D) treatment improves HGPS phenotypes, including nuclear morphological abnormalities, DNA repair defects, and premature senescence. Importantly, we discovered that the 1,25D/VDR axis regulates LMNA gene expression, as well as expression of DNA repair factors. 1,25D dramatically reduces progerin production in HGPS cells, while stabilizing BRCA1 and 53BP1, two key factors for genome integrity. Vitamin D/VDR axis emerges as a new target for treatment of HGPS and potentially other lamin-related diseases exhibiting VDR deficiency and genomic instability. Because progerin expression increases with age, maintaining vitamin D/VDR signaling could keep the levels of progerin in check during physiological aging.
Keywords: DNA repair; Gerotarget; genomic instability; laminopathies; progeria; vitamin D receptor.
Conflict of interest statement
All other authors have no conflict of interest.
Figures

Similar articles
-
Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations.Ageing Res Rev. 2017 Jan;33:18-29. doi: 10.1016/j.arr.2016.06.007. Epub 2016 Jun 29. Ageing Res Rev. 2017. PMID: 27374873 Free PMC article. Review.
-
Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson-Gilford progeria syndrome.Med Hypotheses. 2018 Sep;118:151-162. doi: 10.1016/j.mehy.2018.06.029. Epub 2018 Jun 28. Med Hypotheses. 2018. PMID: 30037605
-
Towards delineating the chain of events that cause premature senescence in the accelerated aging syndrome Hutchinson-Gilford progeria (HGPS).Biochem Soc Trans. 2020 Jun 30;48(3):981-991. doi: 10.1042/BST20190882. Biochem Soc Trans. 2020. PMID: 32539085 Free PMC article. Review.
-
Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture.J Proteomics. 2013 Oct 8;91:466-77. doi: 10.1016/j.jprot.2013.08.008. Epub 2013 Aug 20. J Proteomics. 2013. PMID: 23969228
-
Progerin-Induced Replication Stress Facilitates Premature Senescence in Hutchinson-Gilford Progeria Syndrome.Mol Cell Biol. 2017 Jun 29;37(14):e00659-16. doi: 10.1128/MCB.00659-16. Print 2017 Jul 15. Mol Cell Biol. 2017. PMID: 28483909 Free PMC article.
Cited by
-
Metabolic Dysfunction in Hutchinson-Gilford Progeria Syndrome.Cells. 2020 Feb 8;9(2):395. doi: 10.3390/cells9020395. Cells. 2020. PMID: 32046343 Free PMC article. Review.
-
Farnesyltransferase inhibitor and rapamycin correct aberrant genome organisation and decrease DNA damage respectively, in Hutchinson-Gilford progeria syndrome fibroblasts.Biogerontology. 2018 Dec;19(6):579-602. doi: 10.1007/s10522-018-9758-4. Epub 2018 Jun 15. Biogerontology. 2018. PMID: 29907918 Free PMC article.
-
The role of prelamin A post-translational maturation in stress response and 53BP1 recruitment.Front Cell Dev Biol. 2022 Nov 16;10:1018102. doi: 10.3389/fcell.2022.1018102. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467410 Free PMC article.
-
Doubled lifespan and patient-like pathologies in progeria mice fed high-fat diet.Aging Cell. 2019 Feb;18(1):e12852. doi: 10.1111/acel.12852. Epub 2018 Dec 12. Aging Cell. 2019. PMID: 30548460 Free PMC article.
-
Pharmacotherapy to gene editing: potential therapeutic approaches for Hutchinson-Gilford progeria syndrome.Geroscience. 2020 Apr;42(2):467-494. doi: 10.1007/s11357-020-00167-3. Epub 2020 Feb 11. Geroscience. 2020. PMID: 32048129 Free PMC article. Review.
References
-
- Pereira S, Bourgeois P, Navarro C, Esteves-Vieira V, Cau P, De Sandre-Giovannoli A, Levy N. HGPS and related premature aging disorders: from genomic identification to the first therapeutic approaches. Mech Ageing Dev. 2008;129:449–459. - PubMed
-
- Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, Gerhard-Herman M, Machan JT, Kutys R, Virmani R, Collins FS, Wight TN, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. - PMC - PubMed
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Levy N. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. - PubMed
-
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

