Mnn10 Maintains Pathogenicity in Candida albicans by Extending α-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity
- PMID: 27144456
- PMCID: PMC4856274
- DOI: 10.1371/journal.ppat.1005617
Mnn10 Maintains Pathogenicity in Candida albicans by Extending α-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity
Abstract
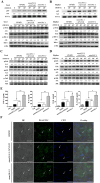
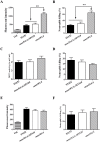
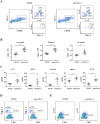
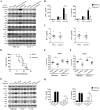
The cell wall is a dynamic structure that is important for the pathogenicity of Candida albicans. Mannan, which is located in the outermost layer of the cell wall, has been shown to contribute to the pathogenesis of C. albicans, however, the molecular mechanism by which this occurs remains unclear. Here we identified a novel α-1,6-mannosyltransferase encoded by MNN10 in C. albicans. We found that Mnn10 is required for cell wall α-1,6-mannose backbone biosynthesis and polysaccharides organization. Deletion of MNN10 resulted in significant attenuation of the pathogenesis of C. albicans in a murine systemic candidiasis model. Inhibition of α-1,6-mannose backbone extension did not, however, impact the invasive ability of C. albicans in vitro. Notably, mnn10 mutant restored the invasive capacity in athymic nude mice, which further supports the notion of an enhanced host antifungal defense related to this backbone change. Mnn10 mutant induced enhanced Th1 and Th17 cell mediated antifungal immunity, and resulted in enhanced recruitment of neutrophils and monocytes for pathogen clearance in vivo. We also demonstrated that MNN10 could unmask the surface β-(1,3)-glucan, a crucial pathogen-associated molecular pattern (PAMP) of C. albicans recognized by host Dectin-1. Our results demonstrate that mnn10 mutant could stimulate an enhanced Dectin-1 dependent immune response of macrophages in vitro, including the activation of nuclear factor-κB, mitogen-activated protein kinase pathways, and secretion of specific cytokines such as TNF-α, IL-6, IL-1β and IL-12p40. In summary, our study indicated that α-1,6-mannose backbone is critical for the pathogenesis of C. albicans via shielding β-glucan from recognition by host Dectin-1 mediated immune recognition. Moreover, our work suggests that inhibition of α-1,6-mannose extension by Mnn10 may represent a novel modality to reduce the pathogenicity of C. albicans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Abolishing Cell Wall Glycosylphosphatidylinositol-Anchored Proteins in Candida albicans Enhances Recognition by Host Dectin-1.Infect Immun. 2015 Jul;83(7):2694-704. doi: 10.1128/IAI.00097-15. Epub 2015 Apr 20. Infect Immun. 2015. PMID: 25895969 Free PMC article.
-
The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans.PLoS Pathog. 2013;9(4):e1003276. doi: 10.1371/journal.ppat.1003276. Epub 2013 Apr 25. PLoS Pathog. 2013. PMID: 23633946 Free PMC article.
-
Histone deacetylase Sir2 promotes the systemic Candida albicans infection by facilitating its immune escape via remodeling the cell wall and maintaining the metabolic activity.mBio. 2024 Jun 12;15(6):e0044524. doi: 10.1128/mbio.00445-24. Epub 2024 Apr 29. mBio. 2024. PMID: 38682948 Free PMC article.
-
Dressed to impress: impact of environmental adaptation on the Candida albicans cell wall.Mol Microbiol. 2015 Jul;97(1):7-17. doi: 10.1111/mmi.13020. Epub 2015 May 9. Mol Microbiol. 2015. PMID: 25846717 Free PMC article. Review.
-
Mannosylation in Candida albicans: role in cell wall function and immune recognition.Mol Microbiol. 2013 Dec;90(6):1147-61. doi: 10.1111/mmi.12426. Epub 2013 Nov 8. Mol Microbiol. 2013. PMID: 24125554 Free PMC article. Review.
Cited by
-
Role of Protein Glycosylation in Interactions of Medically Relevant Fungi with the Host.J Fungi (Basel). 2021 Oct 18;7(10):875. doi: 10.3390/jof7100875. J Fungi (Basel). 2021. PMID: 34682296 Free PMC article. Review.
-
Control of β-glucan exposure by the endo-1,3-glucanase Eng1 in Candida albicans modulates virulence.PLoS Pathog. 2022 Jan 7;18(1):e1010192. doi: 10.1371/journal.ppat.1010192. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 34995333 Free PMC article.
-
Accessibility and contribution to glucan masking of natural and genetically tagged versions of yeast wall protein 1 of Candida albicans.PLoS One. 2018 Jan 12;13(1):e0191194. doi: 10.1371/journal.pone.0191194. eCollection 2018. PLoS One. 2018. PMID: 29329339 Free PMC article.
-
Preventing Candida albicans from subverting host plasminogen for invasive infection treatment.Emerg Microbes Infect. 2020 Dec;9(1):2417-2432. doi: 10.1080/22221751.2020.1840927. Emerg Microbes Infect. 2020. PMID: 33115324 Free PMC article.
-
The secretory Candida effector Sce1 licenses fungal virulence by masking the immunogenic β-1,3-glucan and promoting apoptosis of the host cells.mLife. 2023 Jun 26;2(2):159-177. doi: 10.1002/mlf2.12066. eCollection 2023 Jun. mLife. 2023. PMID: 38817625 Free PMC article.
References
-
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008; 6: 67–78. - PubMed
-
- Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med. 2009; 37: 1612–1618. 10.1097/CCM.0b013e31819efac0 - DOI - PubMed
-
- Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents. 2014; 43: 78–81. 10.1016/j.ijantimicag.2013.09.005 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

