Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression
- PMID: 27144433
- PMCID: PMC5085222
- DOI: 10.18632/oncotarget.9106
Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression
Abstract
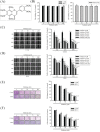
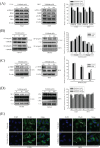
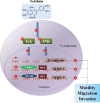
Nobiletin, a polymethoxyflavone, has a few pharmacological activities, including anti-inflammation and anti-cancer effects. However, its effect on human osteosarcoma progression remains uninvestigated. Therefore, we examined the effectiveness of nobiletin against cellular metastasis of human osteosarcoma and the underlying mechanisms. Nobiletin, up to 100 μM without cytotoxicity, significantly decreased motility, migration and invasion as well as enzymatic activities, protein levels and mRNA expressions of matrix metalloproteinase (MMP)-2 and MMP-9 in U2OS and HOS cells. In addition to inhibition of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK), the inhibitory effect of nobiletin on the DNA-binding activity of the transcription factor nuclear factor-kappa B (NF-κB), cAMP response element-binding protein (CREB), and specificity protein 1 (SP-1) in U2OS and HOS cells. Co-treatment with ERK and JNK inhibitors and nobiletin further reduced U2OS cells migration and invasion. These results indicated that nobiletin inhibits human osteosarcoma U2OS and HOS cells motility, migration and invasion by down-regulating MMP-2 and MMP-9 expressions via ERK and JNK pathways and through the inactivation of downstream NF-κB, CREB, and SP-1. Nobiletin has the potential to serve as an anti-metastatic agent for treating osteosarcoma.
Keywords: CREB; MMP; SP-1; metastasis; nobiletin.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures



Similar articles
-
Bufalin-inhibited migration and invasion in human osteosarcoma U-2 OS cells is carried out by suppression of the matrix metalloproteinase-2, ERK, and JNK signaling pathways.Environ Toxicol. 2014 Jan;29(1):21-9. doi: 10.1002/tox.20769. Epub 2011 Sep 16. Environ Toxicol. 2014. PMID: 21922632
-
Astrocyte elevated gene-1 (AEG-1) promotes osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway.Biochem Biophys Res Commun. 2014 Oct 3;452(4):933-9. doi: 10.1016/j.bbrc.2014.09.009. Epub 2014 Sep 6. Biochem Biophys Res Commun. 2014. PMID: 25204501
-
Tricetin inhibits human osteosarcoma cells metastasis by transcriptionally repressing MMP-9 via p38 and Akt pathways.Environ Toxicol. 2017 Aug;32(8):2032-2040. doi: 10.1002/tox.22380. Epub 2016 Nov 8. Environ Toxicol. 2017. PMID: 27860196
-
Recent advances in the therapeutic potential of nobiletin against respiratory diseases.Phytomedicine. 2024 Jun;128:155506. doi: 10.1016/j.phymed.2024.155506. Epub 2024 Mar 1. Phytomedicine. 2024. PMID: 38522319 Review.
-
Nobiletin in Cancer Therapy; Mechanisms and Therapy Perspectives.Curr Pharm Des. 2023;29(22):1713-1728. doi: 10.2174/1381612829666230426115424. Curr Pharm Des. 2023. PMID: 37185325 Review.
Cited by
-
Hesperetin Inhibits TGF-β1-Induced Migration and Invasion of Triple Negative Breast Cancer MDA-MB-231 Cells via Suppressing Fyn/Paxillin/RhoA Pathway.Integr Cancer Ther. 2022 Jan-Dec;21:15347354221086900. doi: 10.1177/15347354221086900. Integr Cancer Ther. 2022. PMID: 35297710 Free PMC article.
-
Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies.MedComm (2020). 2023 Jul 10;4(4):e308. doi: 10.1002/mco2.308. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37441462 Free PMC article. Review.
-
Expression and functional analysis of the nobiletin biosynthesis-related gene CitOMT in citrus fruit.Sci Rep. 2020 Sep 17;10(1):15288. doi: 10.1038/s41598-020-72277-z. Sci Rep. 2020. PMID: 32943728 Free PMC article.
-
The Application of Citrus folium in Breast Cancer and the Mechanism of Its Main Component Nobiletin: A Systematic Review.Evid Based Complement Alternat Med. 2021 Jun 29;2021:2847466. doi: 10.1155/2021/2847466. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34257674 Free PMC article. Review.
-
Melatonin as a potential inhibitory agent in head and neck cancer.Oncotarget. 2017 Aug 9;8(52):90545-90556. doi: 10.18632/oncotarget.20079. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163852 Free PMC article. Review.
References
-
- Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pacific journal of cancer prevention: APJCP. 2014;15:1085–1091. - PubMed
-
- Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. - PubMed
-
- Foroni C, Broggini M, Generali D, Damia G. Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer treatment reviews. 2012;38:689–697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

