Clinical iron deficiency disturbs normal human responses to hypoxia
- PMID: 27140401
- PMCID: PMC4887172
- DOI: 10.1172/JCI85715
Clinical iron deficiency disturbs normal human responses to hypoxia
Abstract
Background: Iron bioavailability has been identified as a factor that influences cellular hypoxia sensing, putatively via an action on the hypoxia-inducible factor (HIF) pathway. We therefore hypothesized that clinical iron deficiency would disturb integrated human responses to hypoxia.
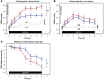
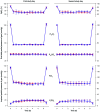
Methods: We performed a prospective, controlled, observational study of the effects of iron status on hypoxic pulmonary hypertension. Individuals with absolute iron deficiency (ID) and an iron-replete (IR) control group were exposed to two 6-hour periods of isocapnic hypoxia. The second hypoxic exposure was preceded by i.v. infusion of iron. Pulmonary artery systolic pressure (PASP) was serially assessed with Doppler echocardiography.
Results: Thirteen ID individuals completed the study and were age- and sex-matched with controls. PASP did not differ by group or study day before each hypoxic exposure. During the first 6-hour hypoxic exposure, the rise in PASP was 6.2 mmHg greater in the ID group (absolute rises 16.1 and 10.7 mmHg, respectively; 95% CI for difference, 2.7-9.7 mmHg, P = 0.001). Intravenous iron attenuated the PASP rise in both groups; however, the effect was greater in ID participants than in controls (absolute reductions 11.1 and 6.8 mmHg, respectively; 95% CI for difference in change, -8.3 to -0.3 mmHg, P = 0.035). Serum erythropoietin responses to hypoxia also differed between groups.
Conclusion: Clinical iron deficiency disturbs normal responses to hypoxia, as evidenced by exaggerated hypoxic pulmonary hypertension that is reversed by subsequent iron administration. Disturbed hypoxia sensing and signaling provides a mechanism through which iron deficiency may be detrimental to human health.
Trial registration: ClinicalTrials.gov (NCT01847352).
Funding: M.C. Frise is the recipient of a British Heart Foundation Clinical Research Training Fellowship (FS/14/48/30828). K.L. Dorrington is supported by the Dunhill Medical Trust (R178/1110). D.J. Roberts was supported by R&D funding from National Health Service (NHS) Blood and Transplant and a National Institute for Health Research (NIHR) Programme grant (RP-PG-0310-1004). This research was funded by the NIHR Oxford Biomedical Research Centre Programme.
Figures
Similar articles
-
Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials.JAMA. 2009 Oct 7;302(13):1444-50. doi: 10.1001/jama.2009.1404. JAMA. 2009. PMID: 19809026 Clinical Trial.
-
Exaggerated pulmonary vascular response to acute hypoxia in older men.Exp Physiol. 2015 Oct;100(10):1187-98. doi: 10.1113/EP085403. Epub 2015 Sep 14. Exp Physiol. 2015. PMID: 26260891
-
The increase in pulmonary arterial pressure caused by hypoxia depends on iron status.J Physiol. 2008 Dec 15;586(24):5999-6005. doi: 10.1113/jphysiol.2008.160960. Epub 2008 Oct 27. J Physiol. 2008. PMID: 18955380 Free PMC article. Clinical Trial.
-
Limitations and strengths of doppler/echo pulmonary artery systolic pressure-right heart catheterization correlations: a systematic literature review.Echocardiography. 2015 Jan;32(1):10-8. doi: 10.1111/echo.12594. Epub 2014 Mar 25. Echocardiography. 2015. PMID: 24661140 Review.
-
HIF and pulmonary vascular responses to hypoxia.J Appl Physiol (1985). 2014 Apr 1;116(7):867-74. doi: 10.1152/japplphysiol.00643.2013. Epub 2013 Dec 12. J Appl Physiol (1985). 2014. PMID: 24336881 Free PMC article. Review.
Cited by
-
Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains.J Clin Invest. 2018 Aug 31;128(9):3704-3715. doi: 10.1172/JCI120847. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 30080181 Free PMC article. Review.
-
Impact of serum iron levels on in-hospital mortality and clinical outcomes in patients with ST segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention: a retrospective analysis.Coron Artery Dis. 2024 Nov 1;35(7):539-546. doi: 10.1097/MCA.0000000000001393. Epub 2024 May 30. Coron Artery Dis. 2024. PMID: 38809141 Free PMC article.
-
Impact of Metal Ions on Cellular Functions: A Focus on Mesenchymal Stem/Stromal Cell Differentiation.Int J Mol Sci. 2024 Sep 20;25(18):10127. doi: 10.3390/ijms251810127. Int J Mol Sci. 2024. PMID: 39337612 Free PMC article. Review.
-
Sleep and Tibialis Anterior Muscle Activity in Mice With Mild Hypoxia and Iron Deficiency: Implications for the Restless Legs Syndrome.Front Physiol. 2018 Dec 17;9:1818. doi: 10.3389/fphys.2018.01818. eCollection 2018. Front Physiol. 2018. PMID: 30618828 Free PMC article.
-
Iron Deficiency as a Therapeutic Target in Cardiovascular Disease.Pharmaceuticals (Basel). 2019 Aug 28;12(3):125. doi: 10.3390/ph12030125. Pharmaceuticals (Basel). 2019. PMID: 31466321 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

