Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network
- PMID: 27138253
- PMCID: PMC4862333
- DOI: 10.1083/jcb.201601089
Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network
Abstract
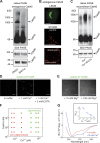
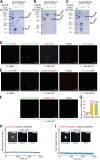
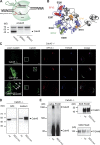
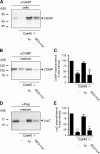
Sorting and export of transmembrane cargoes and lysosomal hydrolases at the trans-Golgi network (TGN) are well understood. However, elucidation of the mechanism by which secretory cargoes are segregated for their release into the extracellular space remains a challenge. We have previously demonstrated that, in a reaction that requires Ca(2+), the soluble TGN-resident protein Cab45 is necessary for the sorting of secretory cargoes at the TGN. Here, we report that Cab45 reversibly assembles into oligomers in the presence of Ca(2+) These Cab45 oligomers specifically bind secretory proteins, such as COMP and LyzC, in a Ca(2+)-dependent manner in vitro. In intact cells, mutation of the Ca(2+)-binding sites in Cab45 impairs oligomerization, as well as COMP and LyzC sorting. Superresolution microscopy revealed that Cab45 colocalizes with secretory proteins and the TGN Ca(2+) pump (SPCA1) in specific TGN microdomains. These findings reveal that Ca(2+)-dependent changes in Cab45 mediate sorting of specific cargo molecules at the TGN.
© 2016 von Blume et al.
Figures
Similar articles
-
Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network.Dev Cell. 2018 Nov 19;47(4):464-478.e8. doi: 10.1016/j.devcel.2018.10.012. Epub 2018 Nov 1. Dev Cell. 2018. PMID: 30393074 Free PMC article.
-
Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network.J Cell Biol. 2012 Dec 24;199(7):1057-66. doi: 10.1083/jcb.201207180. J Cell Biol. 2012. PMID: 23266954 Free PMC article.
-
Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network.Eur J Cell Biol. 2017 Aug;96(5):383-390. doi: 10.1016/j.ejcb.2017.03.001. Epub 2017 Mar 18. Eur J Cell Biol. 2017. PMID: 28372832 Review.
-
Exploring new routes for secretory protein export from the trans-Golgi network.Mol Biol Cell. 2018 Feb 1;29(3):235-240. doi: 10.1091/mbc.E17-02-0117. Mol Biol Cell. 2018. PMID: 29382805 Free PMC article.
-
Secretory cargo sorting at the trans-Golgi network.Trends Cell Biol. 2014 Oct;24(10):584-93. doi: 10.1016/j.tcb.2014.04.007. Epub 2014 May 16. Trends Cell Biol. 2014. PMID: 24841758 Review.
Cited by
-
GARP dysfunction results in COPI displacement, depletion of Golgi v-SNAREs and calcium homeostasis proteins.Front Cell Dev Biol. 2022 Dec 12;10:1066504. doi: 10.3389/fcell.2022.1066504. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578782 Free PMC article.
-
Calcium flow at ER-TGN contact sites facilitates secretory cargo export.Mol Biol Cell. 2024 Apr 1;35(4):ar50. doi: 10.1091/mbc.E23-03-0099. Epub 2024 Jan 31. Mol Biol Cell. 2024. PMID: 38294859 Free PMC article.
-
Primary Active Ca2+ Transport Systems in Health and Disease.Cold Spring Harb Perspect Biol. 2020 Feb 3;12(2):a035113. doi: 10.1101/cshperspect.a035113. Cold Spring Harb Perspect Biol. 2020. PMID: 31501194 Free PMC article. Review.
-
Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network.Dev Cell. 2018 Nov 19;47(4):464-478.e8. doi: 10.1016/j.devcel.2018.10.012. Epub 2018 Nov 1. Dev Cell. 2018. PMID: 30393074 Free PMC article.
-
A genetically encoded toolkit of functionalized nanobodies against fluorescent proteins for visualizing and manipulating intracellular signalling.BMC Biol. 2019 May 23;17(1):41. doi: 10.1186/s12915-019-0662-4. BMC Biol. 2019. PMID: 31122229 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

