Unravelling the Actin Cytoskeleton: A New Competitive Edge?
- PMID: 27133808
- PMCID: PMC4961066
- DOI: 10.1016/j.tcb.2016.04.001
Unravelling the Actin Cytoskeleton: A New Competitive Edge?
Abstract
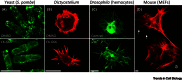
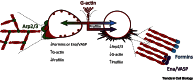
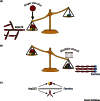
Dynamic rearrangements in the actin cytoskeleton underlie a wide range of cell behaviours, which in turn contribute to many aspects of human health including embryogenesis, cancer metastasis, wound healing, and inflammation. Precise control of the actin cytoskeleton requires the coordinated activity of a diverse set of different actin regulators. However, our current understanding of the actin cytoskeleton has focused on how individual actin regulatory pathways function in isolation from one another. Recently, competition has emerged as a means by which different actin assembly factors can influence each other's activity at the cellular level. Here such findings will be used to explore the possibility that competition within the actin cytoskeleton confers cellular plasticity and the ability to prioritise multiple conflicting stimuli.
Keywords: actin; chemotaxis; competition; cytoskeleton; filopod; lamellipod.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Modulation of T cell signaling by the actin cytoskeleton.J Cell Sci. 2013 Mar 1;126(Pt 5):1049-58. doi: 10.1242/jcs.098210. J Cell Sci. 2013. PMID: 23620508 Review.
-
Plasticity of the actin cytoskeleton in response to extracellular matrix nanostructure and dimensionality.Biochem Soc Trans. 2014 Oct;42(5):1356-66. doi: 10.1042/BST20140139. Biochem Soc Trans. 2014. PMID: 25233415
-
The yeast actin cytoskeleton.FEMS Microbiol Rev. 2014 Mar;38(2):213-27. doi: 10.1111/1574-6976.12064. Epub 2014 Feb 20. FEMS Microbiol Rev. 2014. PMID: 24467403 Review.
-
Capping protein: new insights into mechanism and regulation.Trends Biochem Sci. 2004 Aug;29(8):418-28. doi: 10.1016/j.tibs.2004.06.003. Trends Biochem Sci. 2004. PMID: 15362226 Review.
-
Internetwork competition for monomers governs actin cytoskeleton organization.Nat Rev Mol Cell Biol. 2016 Dec;17(12):799-810. doi: 10.1038/nrm.2016.106. Epub 2016 Sep 14. Nat Rev Mol Cell Biol. 2016. PMID: 27625321 Free PMC article.
Cited by
-
Cyclosporine A Modulates LSP1 Protein Levels in Human B Cells to Attenuate B Cell Migration at Low O2 Levels.Life (Basel). 2022 Aug 22;12(8):1284. doi: 10.3390/life12081284. Life (Basel). 2022. PMID: 36013463 Free PMC article.
-
WASP family proteins and formins compete in pseudopod- and bleb-based migration.J Cell Biol. 2018 Feb 5;217(2):701-714. doi: 10.1083/jcb.201705160. Epub 2017 Nov 30. J Cell Biol. 2018. PMID: 29191847 Free PMC article.
-
Unified control of amoeboid pseudopod extension in multiple organisms by branched F-actin in the front and parallel F-actin/myosin in the cortex.PLoS One. 2020 Dec 9;15(12):e0243442. doi: 10.1371/journal.pone.0243442. eCollection 2020. PLoS One. 2020. PMID: 33296414 Free PMC article.
-
Flow-Through Optical Chromatography in Combination with Confocal Raman Microspectroscopy: A Novel Label-Free Approach To Detect Responses of Live Macrophages to Environmental Stimuli.ACS Omega. 2019 Jul 31;4(7):12938-12947. doi: 10.1021/acsomega.9b01162. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460420 Free PMC article.
-
Persistent inhibition of pore-based cell migration by sub-toxic doses of miuraenamide, an actin filament stabilizer.Sci Rep. 2017 Nov 27;7(1):16407. doi: 10.1038/s41598-017-16759-7. Sci Rep. 2017. PMID: 29180826 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

