The Atypical Inhibitor of NF-κB, IκBζ, Controls Macrophage Interleukin-10 Expression
- PMID: 27129283
- PMCID: PMC4933459
- DOI: 10.1074/jbc.M116.718825
The Atypical Inhibitor of NF-κB, IκBζ, Controls Macrophage Interleukin-10 Expression
Abstract
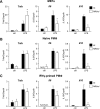
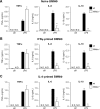
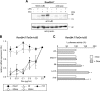
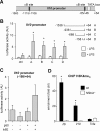
Macrophages constitute a first line of pathogen defense by triggering a number of inflammatory responses and the secretion of various pro-inflammatory cytokines. Recently, we and others found that IκBζ, an atypical IκB family member and transcriptional coactivator of selected NF-κB target genes, is essential for macrophage expression of a subset of pro-inflammatory cytokines, such as IL-6, IL-12, and CCL2. Despite defective pro-inflammatory cytokine expression, however, IκBζ-deficient mice develop symptoms of chronic inflammation. To elucidate this discrepancy, we analyzed a regulatory role of IκBζ for the expression of anti-inflammatory cytokines and identified IκBζ as an essential activator of IL-10 expression. LPS-challenged peritoneal and bone marrow-derived macrophages from IκBζ-deficient mice revealed strongly decreased transcription and secretion of IL-10 compared with wild-type mice. Moreover, ectopic expression of IκBζ was sufficient to stimulate Il10 transcription. On the molecular level, IκBζ directly activated the Il10 promoter at a proximal κB site and was required for the transcription-enhancing trimethylation of histone 3 at lysine 4. Together, our findings show for the first time the IκBζ-dependent expression of an anti-inflammatory cytokine that is crucial in controlling immune responses.
Keywords: NF-κB; NF-κB transcription factor; immunosuppressor; interleukin; macrophage.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
IκBζ is a transcriptional key regulator of CCL2/MCP-1.J Immunol. 2013 May 1;190(9):4812-20. doi: 10.4049/jimmunol.1300089. Epub 2013 Apr 1. J Immunol. 2013. PMID: 23547114
-
IκBζ is a key transcriptional regulator of IL-36-driven psoriasis-related gene expression in keratinocytes.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):10088-10093. doi: 10.1073/pnas.1801377115. Epub 2018 Sep 17. Proc Natl Acad Sci U S A. 2018. PMID: 30224457 Free PMC article.
-
Sodium azide suppresses LPS-induced expression MCP-1 through regulating IκBζ and STAT1 activities in macrophages.Cell Immunol. 2017 May;315:64-70. doi: 10.1016/j.cellimm.2017.02.007. Epub 2017 Apr 4. Cell Immunol. 2017. PMID: 28391993
-
Emerging role of IκBζ in inflammation: Emphasis on psoriasis.Clin Transl Med. 2022 Oct;12(10):e1032. doi: 10.1002/ctm2.1032. Clin Transl Med. 2022. PMID: 36245291 Free PMC article. Review.
-
The central inflammatory regulator IκBζ: induction, regulation and physiological functions.Front Immunol. 2023 Jun 12;14:1188253. doi: 10.3389/fimmu.2023.1188253. eCollection 2023. Front Immunol. 2023. PMID: 37377955 Free PMC article. Review.
Cited by
-
Exclusive Temporal Stimulation of IL-10 Expression in LPS-Stimulated Mouse Macrophages by cAMP Inducers and Type I Interferons.Front Immunol. 2019 Aug 6;10:1788. doi: 10.3389/fimmu.2019.01788. eCollection 2019. Front Immunol. 2019. PMID: 31447835 Free PMC article.
-
MiR-25 blunts autophagy and promotes the survival of Mycobacterium tuberculosis by regulating NPC1.iScience. 2022 Apr 22;25(5):104279. doi: 10.1016/j.isci.2022.104279. eCollection 2022 May 20. iScience. 2022. PMID: 35586071 Free PMC article.
-
Simultaneous quantification of DNA damage and mitochondrial copy number by long-run DNA-damage quantification (LORD-Q).Oncotarget. 2017 Aug 10;8(68):112417-112425. doi: 10.18632/oncotarget.20112. eCollection 2017 Dec 22. Oncotarget. 2017. PMID: 29348835 Free PMC article.
-
Compromised Anti-inflammatory Action of Neutrophil Extracellular Traps in PAD4-Deficient Mice Contributes to Aggravated Acute Inflammation After Myocardial Infarction.Front Immunol. 2019 Oct 1;10:2313. doi: 10.3389/fimmu.2019.02313. eCollection 2019. Front Immunol. 2019. PMID: 31632398 Free PMC article.
-
Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy.Signal Transduct Target Ther. 2021 Oct 20;6(1):367. doi: 10.1038/s41392-021-00764-4. Signal Transduct Target Ther. 2021. PMID: 34667157 Free PMC article. Review.
References
-
- Gordon S., and Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 - PubMed
-
- Ginhoux F., and Jung S. (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol 14, 392–404 - PubMed
-
- Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

