Structural and Functional Characterization of the Major Allergen Amb a 11 from Short Ragweed Pollen
- PMID: 27129273
- PMCID: PMC4933224
- DOI: 10.1074/jbc.M115.702001
Structural and Functional Characterization of the Major Allergen Amb a 11 from Short Ragweed Pollen
Abstract
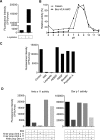
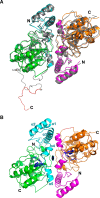
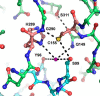
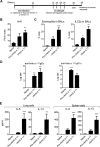
Allergy to the short ragweed (Ambrosia artemisiifolia) pollen is a major health problem. The ragweed allergen repertoire has been recently expanded with the identification of Amb a 11, a new major allergen belonging to the cysteine protease family. To better characterize Amb a 11, a recombinant proform of the molecule with a preserved active site was produced in Escherichia coli, refolded, and processed in vitro into a mature enzyme. The enzymatic activity is revealed by maturation following an autocatalytic processing resulting in the cleavage of both N- and C-terminal propeptides. The 2.05-Å resolution crystal structure of pro-Amb a 11 shows an overall typical C1A cysteine protease fold with a network of molecular interactions between the N-terminal propeptide and the catalytic triad of the enzyme. The allergenicity of Amb a 11 was confirmed in a murine sensitization model, resulting in airway inflammation, production of serum IgEs, and induction of Th2 immune responses. Of note, inflammatory responses were higher with the mature form, demonstrating that the cysteine protease activity critically contributes to the allergenicity of the molecule. Collectively, our results clearly demonstrate that Amb a 11 is a bona fide cysteine protease exhibiting a strong allergenicity. As such, it should be considered as an important molecule for diagnosis and immunotherapy of ragweed pollen allergy.
Keywords: allergen; cysteine protease; immunotherapy; protein processing; ragweed; structure-function.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Identification of the cysteine protease Amb a 11 as a novel major allergen from short ragweed.J Allergy Clin Immunol. 2015 Oct;136(4):1055-64. doi: 10.1016/j.jaci.2015.03.001. Epub 2015 Apr 10. J Allergy Clin Immunol. 2015. PMID: 25865353 Clinical Trial.
-
A new cysteine protease allergen from Ambrosia trifida pollen: proforms and mature forms.Mol Immunol. 2022 Jul;147:170-179. doi: 10.1016/j.molimm.2022.05.003. Epub 2022 May 19. Mol Immunol. 2022. PMID: 35598503
-
Non-Specific Lipid Transfer Protein Amb a 6 Is a Source-Specific Important Allergenic Molecule in Ragweed Pollen.Int J Mol Sci. 2024 Jun 13;25(12):6513. doi: 10.3390/ijms25126513. Int J Mol Sci. 2024. PMID: 38928218 Free PMC article.
-
New insights into ragweed pollen allergens.Curr Allergy Asthma Rep. 2015 Nov;15(11):63. doi: 10.1007/s11882-015-0565-6. Curr Allergy Asthma Rep. 2015. PMID: 26383916 Review.
-
Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe.Int Arch Allergy Immunol. 2018;176(3-4):163-180. doi: 10.1159/000487997. Epub 2018 May 22. Int Arch Allergy Immunol. 2018. PMID: 29788026 Review.
Cited by
-
Identification of Proteases and Protease Inhibitors in Allergenic and Non-Allergenic Pollen.Int J Mol Sci. 2017 Jun 5;18(6):1199. doi: 10.3390/ijms18061199. Int J Mol Sci. 2017. PMID: 28587253 Free PMC article.
-
Isolation and characterization of a tandem-repeated cysteine protease from the symbiotic dinoflagellate Symbiodinium sp. KB8.PLoS One. 2019 Jan 31;14(1):e0211534. doi: 10.1371/journal.pone.0211534. eCollection 2019. PLoS One. 2019. PMID: 30703144 Free PMC article.
-
Design of an Epitope-Based Peptide Vaccine Against the Major Allergen Amb a 11 Using Immunoinformatic Approaches.Protein J. 2022 Apr;41(2):315-326. doi: 10.1007/s10930-022-10050-z. Epub 2022 Apr 1. Protein J. 2022. PMID: 35362839 Free PMC article.
-
Initiating pollen sensitization - complex source, complex mechanisms.Clin Transl Allergy. 2020 Aug 31;10:36. doi: 10.1186/s13601-020-00341-y. eCollection 2020. Clin Transl Allergy. 2020. PMID: 32884636 Free PMC article. Review.
-
Cari p 1, a Novel Polygalacturonase Allergen From Papaya Acting as Respiratory and Food Sensitizer.Front Plant Sci. 2018 Jun 18;9:823. doi: 10.3389/fpls.2018.00823. eCollection 2018. Front Plant Sci. 2018. PMID: 29967633 Free PMC article.
References
-
- Arbes S. J. Jr., Gergen P. J., Elliott L., and Zeldin D. C. (2005) Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 116, 377–383 - PubMed
-
- D'Amato G., Cecchi L., Bonini S., Nunes C., Annesi-Maesano I., Behrendt H., Liccardi G., Popov T., and van Cauwenberge P. (2007) Allergenic pollen and pollen allergy in Europe. Allergy 62, 976–990 - PubMed
-
- Burbach G. J., Heinzerling L. M., Röhnelt C., Bergmann K. C., Behrendt H., Zuberbier T., and GA(2)LEN study (2009) Ragweed sensitization in Europe—GA(2)LEN study suggests increasing prevalence. Allergy 64, 664–665 - PubMed
-
- Smith M., Cecchi L., Skjøth C. A., Karrer G., and Šikoparija B. (2013) Common ragweed: a threat to environmental health in Europe. Environ. Int. 61, 115–126 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

