COMMD7 is correlated with a novel NF-κB positive feedback loop in hepatocellular carcinoma
- PMID: 27129158
- PMCID: PMC5078050
- DOI: 10.18632/oncotarget.9047
COMMD7 is correlated with a novel NF-κB positive feedback loop in hepatocellular carcinoma
Abstract
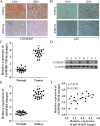
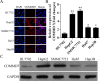
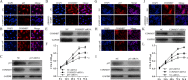
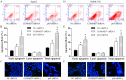
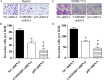
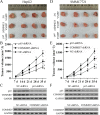
The correlation between nuclear factor-kappa B (NF-κB) and COMMD7 in hepatocellular carcinoma (HCC) development remained unclear. Here, our clinicopathological data showed that COMMD7 is overexpressed in HCC with a correlation to NF-κB. Using HepG2 and SMMC-7721 cells that aberrantly overexpressed COMMD7, we found that NF-κB directly binds with COMMD7 promoter and serves as an activator for COMMD7 transcription by luciferase reporter assay, chromatin immunoprecipitation (ChIP), and electrophoretic mobility shift assay (EMSA). In both HepG2 cells and SMMC-7721 cells, the silencing of COMMD7 significantly inhibited the cell proliferation, whereas NF-κB silencing inhibited the expression of COMMD7 and further inhibited cell proliferation. In addition, cell apoptosis was promoted by COMMD7 silencing, and further promoted by NF-κB silencing. Cell migration and invasion were also inhibited by COMMD7 silencing, and further inhibited by NF-κB silencing. Thus, COMMD7 is correlated with a novel NF-κB positive feedback loop in hepatocellular carcinoma. Developing strategies for the treatment of HCC should consider the correlation between NF-κB and COMMD7, so as to improve the specificity and sensitivity of therapy and to reduce toxicity.
Keywords: COMMD7; apoptosis; hepatocellular carcinoma; nuclear factor-kappa B; proliferation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
ShRNA-targeted COMMD7 suppresses hepatocellular carcinoma growth.PLoS One. 2012;7(9):e45412. doi: 10.1371/journal.pone.0045412. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049798 Free PMC article.
-
Eukaryotic elongation factor-1α 2 knockdown inhibits hepatocarcinogenesis by suppressing PI3K/Akt/NF-κB signaling.World J Gastroenterol. 2016 Apr 28;22(16):4226-37. doi: 10.3748/wjg.v22.i16.4226. World J Gastroenterol. 2016. PMID: 27122673 Free PMC article.
-
Yin Yang 1-mediated epigenetic silencing of tumour-suppressive microRNAs activates nuclear factor-κB in hepatocellular carcinoma.J Pathol. 2016 Apr;238(5):651-64. doi: 10.1002/path.4688. J Pathol. 2016. PMID: 26800240
-
Multifaceted role of NF-κB in hepatocellular carcinoma therapy: Molecular landscape, therapeutic compounds and nanomaterial approaches.Environ Res. 2023 Jul 1;228:115767. doi: 10.1016/j.envres.2023.115767. Epub 2023 Mar 24. Environ Res. 2023. PMID: 36966991 Review.
-
Expression and role of icam-1 in the occurrence and development of hepatocellular carcinoma.Asian Pac J Cancer Prev. 2013;14(3):1579-83. doi: 10.7314/apjcp.2013.14.3.1579. Asian Pac J Cancer Prev. 2013. PMID: 23679239 Review.
Cited by
-
COMMD7 Regulates NF-κB Signaling Pathway in Hepatocellular Carcinoma Stem-like Cells.Mol Ther Oncolytics. 2018 Dec 14;12:112-123. doi: 10.1016/j.omto.2018.12.006. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30719501 Free PMC article.
-
Transcriptional analysis of the expression, prognostic value and immune infiltration activities of the COMMD protein family in hepatocellular carcinoma.BMC Cancer. 2021 Sep 7;21(1):1001. doi: 10.1186/s12885-021-08699-3. BMC Cancer. 2021. PMID: 34493238 Free PMC article.
-
Investigating the Association between COMMD3 Expression and the Prognosis of Hepatocellular Carcinoma.J Cancer. 2022 Mar 21;13(6):1871-1881. doi: 10.7150/jca.62454. eCollection 2022. J Cancer. 2022. PMID: 35399735 Free PMC article.
-
High expression of COMMD7 is an adverse prognostic factor in acute myeloid leukemia.Aging (Albany NY). 2021 Apr 23;13(8):11988-12006. doi: 10.18632/aging.202901. Epub 2021 Apr 23. Aging (Albany NY). 2021. PMID: 33891561 Free PMC article.
-
COMMD3 Expression Affects Angiogenesis through the HIF1α/VEGF/NF-κB Signaling Pathway in Hepatocellular Carcinoma In Vitro and In Vivo.Oxid Med Cell Longev. 2022 Sep 2;2022:1655502. doi: 10.1155/2022/1655502. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092163 Free PMC article.
References
-
- Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr Probl Diagn Radiol. 2015 - PubMed
-
- Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. - PubMed
-
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

