Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody
- PMID: 27127172
- PMCID: PMC5058720
- DOI: 10.18632/oncotarget.9003
Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody
Abstract
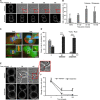
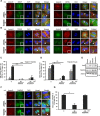
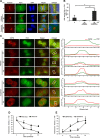
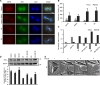
Adhesion to extracellular matrix is required for cell cycle progression through the G1 phase and for the completion of cytokinesis in normal adherent cells. Cancer cells acquire the ability to proliferate anchorage-independently, a characteristic feature of malignantly transformed cells. However, the molecular mechanisms underlying this escape of the normal control mechanisms remain unclear. The current study aimed to identify adhesion-induced reactions regulating the cytokinesis of non-transformed human fibroblasts.The adhesion-dependent control of cytokinesis was found to occur at a late stage close to the abscission, during which the endosomal sorting complex required for transport (ESCRT) severs the thin intercellular bridge connecting two nascent daughter cells. CEP55, a key protein involved in the abscission process, was localized at the midbody in both adherent and non-adherent fibroblasts, but it was unable to efficiently recruit ALIX, TSG101, and consequently the ESCRT-III subunit CHMP4B was missing in the non-adherent cells. PLK1, a kinase that prevents premature recruitment of CEP55 to the midbody, disappeared from this site more rapidly in the non-adherent cells. A FAK-Src signaling pathway downstream of integrin-mediated cell adhesion was found to decelerate both PLK1 degradation and CEP55 accumulation at the midbody. These data identify the regulation of PLK1 and CEP55 as steps where integrins exert control over the cytokinetic abscission.
Keywords: CEP55; FAK; PLK1; cytokinesis; integrin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Tension-induced cytokinetic abscission in human fibroblasts.Oncotarget. 2018 Jan 6;9(10):8999-9009. doi: 10.18632/oncotarget.24016. eCollection 2018 Feb 6. Oncotarget. 2018. PMID: 29507669 Free PMC article.
-
Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis.EMBO J. 2007 Oct 3;26(19):4215-27. doi: 10.1038/sj.emboj.7601850. Epub 2007 Sep 13. EMBO J. 2007. PMID: 17853893 Free PMC article.
-
Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission.J Cell Biol. 2010 Nov 15;191(4):751-60. doi: 10.1083/jcb.201008108. J Cell Biol. 2010. PMID: 21079244 Free PMC article.
-
Knowing when to cut and run: mechanisms that control cytokinetic abscission.Trends Cell Biol. 2013 Sep;23(9):433-41. doi: 10.1016/j.tcb.2013.04.006. Epub 2013 May 22. Trends Cell Biol. 2013. PMID: 23706391 Review.
-
Cytokinesis and cancer: Polo loves ROCK'n' Rho(A).J Genet Genomics. 2010 Mar;37(3):159-72. doi: 10.1016/S1673-8527(09)60034-5. J Genet Genomics. 2010. PMID: 20347825 Review.
Cited by
-
MicroRNA-144-3p inhibits cell proliferation and induces cell apoptosis in prostate cancer by targeting CEP55.Am J Transl Res. 2018 Aug 15;10(8):2457-2468. eCollection 2018. Am J Transl Res. 2018. PMID: 30210684 Free PMC article.
-
Tension-induced cytokinetic abscission in human fibroblasts.Oncotarget. 2018 Jan 6;9(10):8999-9009. doi: 10.18632/oncotarget.24016. eCollection 2018 Feb 6. Oncotarget. 2018. PMID: 29507669 Free PMC article.
-
Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis.Cells. 2022 Apr 16;11(8):1360. doi: 10.3390/cells11081360. Cells. 2022. PMID: 35456039 Free PMC article.
-
[CEP55 may be a potential therapeutic target for non-obstructive azoospermia with maturation arrest].Nan Fang Yi Ke Da Xue Xue Bao. 2019 Sep 30;39(9):1059-1064. doi: 10.12122/j.issn.1673-4254.2019.09.09. Nan Fang Yi Ke Da Xue Xue Bao. 2019. PMID: 31640955 Free PMC article. Chinese.
-
Connections between the cell cycle, cell adhesion and the cytoskeleton.Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180227. doi: 10.1098/rstb.2018.0227. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431178 Free PMC article. Review.
References
-
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. - PubMed
-
- Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014;34:144–153. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

