A Toll-like receptor 2 agonist-fused antigen enhanced antitumor immunity by increasing antigen presentation and the CD8 memory T cells population
- PMID: 27127171
- PMCID: PMC5058719
- DOI: 10.18632/oncotarget.9001
A Toll-like receptor 2 agonist-fused antigen enhanced antitumor immunity by increasing antigen presentation and the CD8 memory T cells population
Abstract
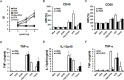
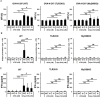
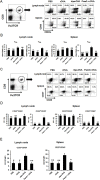
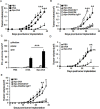
The induction of long-lived effector CD8+ T cells is key to the development of efficient cancer vaccines. In this study, we demonstrated that a Toll-like receptor 2 (TLR2) agonist-fused antigen increased antigen presentation via TLR2 signaling and induced effector memory-like CD8+ T cells against cancer after immunization. The N-terminus of ovalbumin (OVA) was biologically fused with a bacterial lipid moiety TLR2 agonist to produce a recombinant lipidated ovalbumin (rlipo-OVA). We demonstrated that rlipo-OVA activated bone marrow-derived dendritic cells (BM-DCs) maturation and increased antigen presentation by major histocompatibility complex (MHC) class I via TLR2. After immunization, rlipo-OVA skewed the immune response towards T helper (Th) 1 and induced OVA-specific cytotoxic T lymphocyte (CTL) responses. Moreover, immunization with rlipo-OVA induced higher numbers of effector memory (CD44+CD62L-) CD8+ T cells compared with recombinant ovalbumin (rOVA) alone or rOVA mixed with the TLR2 agonist Pam3CSK4. Accordingly, the CD27+CD43+ effector memory CD8+ T cells expressed high levels of the long-lived CD127 marker. The administration of rlipo-OVA could inhibit tumor growth, but the anti-tumor effects were lost after the depletion of CD8 or CD127 cells in vivo. These findings suggested that the TLR2 agonist-fused antigen induced long-lived memory CD8+ T cells for efficient cancer therapy.
Keywords: Toll-like receptor 2; antigen presentation; memory T cells; rlipo-immunogen; tumor regression.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Intradermal injections of polyarginine-containing immunogenic antigens preferentially elicit Tc1 and Th1 activation and antitumour immunity.Br J Dermatol. 2010 Jan;162(1):29-41. doi: 10.1111/j.1365-2133.2009.09490.x. Epub 2009 Oct 26. Br J Dermatol. 2010. PMID: 19863514
-
Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells.Cancer Res. 2001 Nov 1;61(21):7913-9. Cancer Res. 2001. PMID: 11691812
-
Nonspecific CD4(+) T cells with uptake of antigen-specific dendritic cell-released exosomes stimulate antigen-specific CD8(+) CTL responses and long-term T cell memory.J Leukoc Biol. 2007 Oct;82(4):829-38. doi: 10.1189/jlb.0407249. Epub 2007 Jul 11. J Leukoc Biol. 2007. PMID: 17626150
-
Alternative processing pathways for MHC class I-restricted epitope presentation to CD8+ cytotoxic T lymphocytes.Biol Chem Hoppe Seyler. 1994 Nov;375(11):731-6. doi: 10.1515/bchm3.1994.375.11.731. Biol Chem Hoppe Seyler. 1994. PMID: 7535058 Review.
-
Delivery of protein antigens to the immune system by fusion-active virosomes: a comparison with liposomes and ISCOMs.Biosci Rep. 2002 Apr;22(2):323-38. doi: 10.1023/a:1020198908574. Biosci Rep. 2002. PMID: 12428908 Review.
Cited by
-
The Toll-like Receptor 7-Mediated Ro52 Antigen-Presenting Pathway in the Salivary Gland Epithelial Cells of Sjögren's Syndrome.J Clin Med. 2023 Jun 30;12(13):4423. doi: 10.3390/jcm12134423. J Clin Med. 2023. PMID: 37445456 Free PMC article.
-
Peptidoglycan-treated tumor antigen-pulsed dendritic cells impart complete resistance against tumor rechallenge.Clin Exp Immunol. 2020 Sep;201(3):279-288. doi: 10.1111/cei.13468. Epub 2020 Jun 26. Clin Exp Immunol. 2020. PMID: 32443171 Free PMC article.
-
Recombinant lipidated FLIPr effectively enhances mucosal and systemic immune responses for various vaccine types.NPJ Vaccines. 2023 Jun 2;8(1):82. doi: 10.1038/s41541-023-00680-4. NPJ Vaccines. 2023. PMID: 37268688 Free PMC article.
-
SARS-CoV-2 spike-FLIPr fusion protein plus lipidated FLIPr protects against various SARS-CoV-2 variants in hamsters.J Virol. 2024 Feb 20;98(2):e0154623. doi: 10.1128/jvi.01546-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299865 Free PMC article.
-
Efficient Uptake of Recombinant Lipidated Survivin by Antigen-Presenting Cells Initiates Antigen Cross-Presentation and Antitumor Immunity.Front Immunol. 2018 Apr 23;9:822. doi: 10.3389/fimmu.2018.00822. eCollection 2018. Front Immunol. 2018. PMID: 29755461 Free PMC article.
References
-
- Andersen MH, Schrama D, Straten PT, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. - PubMed
-
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4(+) T-cell help controls CD8(+) T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. - PubMed
-
- Filipazzi P, Pilla L, Mariani L, Patuzzo R, Castelli C, Camisaschi C, Maurichi A, Cova A, Rigamonti G, Giardino F, Di Florio A, Asioli M, Frati P, et al. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin Cancer Res. 2012;18:6485–6496. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

