House Dust Mite-Derived Chitin Enhances Th2 Cell Response to Inhaled Allergens, Mainly via a TNF-α-Dependent Pathway
- PMID: 27126730
- PMCID: PMC4853514
- DOI: 10.4168/aair.2016.8.4.362
House Dust Mite-Derived Chitin Enhances Th2 Cell Response to Inhaled Allergens, Mainly via a TNF-α-Dependent Pathway
Abstract
Purpose: Chitin is a potent adjuvant in the development of immune response to inhaled allergens in the airways. According to other studies, chitin is known as multi-faced adjuvants which can induce Th2 responses. Recently, we found that TNF-α is a key mediator in the development of Th2 cell response to inhaled allergens. Here, we evaluated the immunologic mechanisms in the development of airway hypersensitivity to inhaled allergens, enhanced by house dust mite (HDM)-derived chitin.
Methods: The role of TNF-α and TLRs was evaluated in an airway hypersensitivity mouse model induced by a sensitization with an allergen (ovalbumin, OVA) and HDM-derived chitin using mice with the null mutation of target genes.
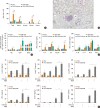
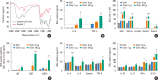
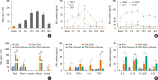
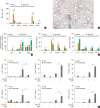
Results: The present study showed that airway sensitization with HDM-derived chitin plus OVA enhanced OVA-induced airway inflammation v. OVA alone. This phenotype was associated with the increased expression of Th1, Th2, and Th17 cytokines and also with the enhanced production of OVA-specific IgE, IgG1, and IgG2a. As for T cell responses, OVA-specific Th2 cell response, enhanced by chitin, was abolished by the treatment of chitinase, whereas Th1 and Th17 cell responses enhanced by this treatment. Moreover, the null mutation of the TNF-α gene revealed similar effects as the chitinase treatment. In contrast, all the OVA-specific T cell responses, enhanced by chitin, were blocked by the absence of TLR2, but not of TLR1, TLR4, or TLR6.
Conclusions: In conclusion, these data suggest that HDM-derived chitin may enhance airway hypersensitivity to inhaled allergens, via the TLR2-dependent pathway, and that chitin-induced TNF-α can be a key mediator in the development of Th2 cell response to inhaled allergens.
Keywords: Chitin; TNF-α; Th2 cell response; house dust mite.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures


Similar articles
-
TNF-alpha is a key mediator in the development of Th2 cell response to inhaled allergens induced by a viral PAMP double-stranded RNA.Allergy. 2012 Sep;67(9):1138-48. doi: 10.1111/j.1398-9995.2012.02871.x. Epub 2012 Jul 5. Allergy. 2012. PMID: 22765163
-
House dust mite allergic airway inflammation facilitates neosensitization to inhaled allergen in mice.Allergy. 2012 Nov;67(11):1383-91. doi: 10.1111/all.12017. Epub 2012 Sep 21. Allergy. 2012. PMID: 22994367
-
Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway.J Immunol. 2007 Sep 1;179(5):2910-7. doi: 10.4049/jimmunol.179.5.2910. J Immunol. 2007. PMID: 17709505
-
Characterization of Innate Immune Responses to House Dust Mite Allergens: Pitfalls and Limitations.Front Allergy. 2021 Mar 11;2:662378. doi: 10.3389/falgy.2021.662378. eCollection 2021. Front Allergy. 2021. PMID: 35386970 Free PMC article. Review.
-
Modeling responses to respiratory house dust mite exposure.Contrib Microbiol. 2007;14:42-67. doi: 10.1159/000107054. Contrib Microbiol. 2007. PMID: 17684332 Review.
Cited by
-
Lactobacillus casei Ghosts as a Vehicle for the Delivery of DNA Vaccines Mediate Immune Responses.Front Immunol. 2022 May 31;13:849409. doi: 10.3389/fimmu.2022.849409. eCollection 2022. Front Immunol. 2022. PMID: 35711427 Free PMC article.
-
TNF is required for TLR ligand-mediated but not protease-mediated allergic airway inflammation.J Clin Invest. 2017 Sep 1;127(9):3313-3326. doi: 10.1172/JCI90890. Epub 2017 Jul 31. J Clin Invest. 2017. PMID: 28758900 Free PMC article.
-
Modulation of Allergic Reactivity in Humans Is Dependent on Schistosoma mansoni Parasite Burden, Low Levels of IL-33 or TNF-α and High Levels of IL-10 in Serum.Front Immunol. 2019 Jan 18;9:3158. doi: 10.3389/fimmu.2018.03158. eCollection 2018. Front Immunol. 2019. PMID: 30713536 Free PMC article.
-
The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size.EMBO Rep. 2018 Dec;19(12):e46065. doi: 10.15252/embr.201846065. Epub 2018 Oct 18. EMBO Rep. 2018. PMID: 30337494 Free PMC article.
-
Chitotriosidase inhibits allergic asthmatic airways via regulation of TGF-β expression and Foxp3+ Treg cells.Allergy. 2018 Aug;73(8):1686-1699. doi: 10.1111/all.13426. Epub 2018 Mar 5. Allergy. 2018. PMID: 29420850 Free PMC article.
References
-
- Dowse GK, Turner KJ, Stewart GA, Alpers MP, Woolcock AJ. The association between Dermatophagoides mites and the increasing prevalence of asthma in village communities within the Papua New Guinea highlands. J Allergy Clin Immunol. 1985;75:75–83. - PubMed
-
- Charpin D, Birnbaum J, Haddi E, Genard G, Lanteaume A, Toumi M, et al. Altitude and allergy to house-dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis. 1991;143:983–986. - PubMed
-
- Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–S24. - PubMed
-
- Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976;21:73–82. - PubMed
-
- Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985;17:93–104. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

