Increased Circulating Levels of Alpha-Ketoglutarate in Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease
- PMID: 27123846
- PMCID: PMC4849715
- DOI: 10.1371/journal.pone.0154601
Increased Circulating Levels of Alpha-Ketoglutarate in Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) causes a wide spectrum of liver damage, ranging from simple steatosis to cirrhosis. However, simple steatosis (SS) and steatohepatitis (NASH) cannot yet be distinguished by clinical or laboratory features. The aim of this study was to assess the relationship between alpha-ketoglutarate and the degrees of NAFLD in morbidly obese patients.
Materials and methods: We used a gas chromatography-quadruple time-of-flight-mass spectrometry analysis to quantify alpha-ketoglutarate in serum from normal-weight subjects (n = 30) and morbidly obese women (n = 97) with or without NAFLD.
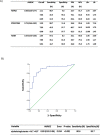
Results: We found that serum levels of alpha-ketoglutarate were significantly higher in morbidly obese women than in normal-weight women. We showed that circulating levels of alpha-ketoglutarate were lower in lean controls and morbidly obese patients without NAFLD. We also found that alpha-ketoglutarate serum levels were higher in both SS and NASH than in normal liver of morbidly obese patients. However, there was no difference between SS and NASH. Moreover, we observed that circulating levels of alpha-ketoglutarate were associated with glucose metabolism parameters, lipid profile, hepatic enzymes and steatosis degree. In addition, diagnostic performance of alpha-ketoglutarate has been analyzed in NAFLD patients. The AUROC curves from patients with liver steatosis exhibited an acceptable clinical utility. Finally, we showed that the combination of biomarkers (AST, ALT and alpha-ketoglutarate) had the highest accuracy in diagnosing liver steatosis.
Conclusion: These findings suggest that alpha-ketoglutarate can determine the presence of non-alcoholic fatty liver in morbidly obese patients but it is not valid a biomarker for NASH.
Conflict of interest statement
Figures


Similar articles
-
Mapping of the circulating metabolome reveals α-ketoglutarate as a predictor of morbid obesity-associated non-alcoholic fatty liver disease.Int J Obes (Lond). 2015 Feb;39(2):279-87. doi: 10.1038/ijo.2014.53. Epub 2014 Mar 28. Int J Obes (Lond). 2015. PMID: 24675715
-
PNPLA3 Expression Is Related to Liver Steatosis in Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2016 Apr 27;17(5):630. doi: 10.3390/ijms17050630. Int J Mol Sci. 2016. PMID: 27128907 Free PMC article.
-
Severity of non-alcoholic fatty liver disease is associated with high systemic levels of tumor necrosis factor alpha and low serum interleukin 10 in morbidly obese patients.Clin Exp Med. 2016 May;16(2):193-202. doi: 10.1007/s10238-015-0347-4. Epub 2015 Apr 18. Clin Exp Med. 2016. PMID: 25894568
-
Novel circulating biomarkers for non-alcoholic fatty liver disease: A systematic review.J Cell Physiol. 2018 Feb;233(2):849-855. doi: 10.1002/jcp.25779. Epub 2017 Mar 28. J Cell Physiol. 2018. PMID: 28063221 Review.
-
Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis.Diabetologia. 2016 Jan;59(1):30-43. doi: 10.1007/s00125-015-3769-3. Epub 2015 Sep 26. Diabetologia. 2016. PMID: 26407715 Review.
Cited by
-
Alpha-ketoglutarate ameliorates abdominal aortic aneurysm via inhibiting PXDN/HOCL/ERK signaling pathways.J Transl Med. 2022 Oct 8;20(1):461. doi: 10.1186/s12967-022-03659-2. J Transl Med. 2022. PMID: 36209172 Free PMC article.
-
Washed microbiota transplantation reduces glycemic variability in unstable diabetes.J Diabetes. 2024 Feb;16(2):e13485. doi: 10.1111/1753-0407.13485. Epub 2023 Oct 17. J Diabetes. 2024. PMID: 37846600 Free PMC article.
-
Discrete Correlation Summation Clustering Reveals Differential Regulation of Liver Metabolism by Thrombospondin-1 in Low-Fat and High-Fat Diet-Fed Mice.Metabolites. 2022 Oct 28;12(11):1036. doi: 10.3390/metabo12111036. Metabolites. 2022. PMID: 36355119 Free PMC article.
-
The most important questions in cancer research and clinical oncology-Question 2-5. Obesity-related cancers: more questions than answers.Chin J Cancer. 2017 Jan 31;36(1):18. doi: 10.1186/s40880-017-0185-8. Chin J Cancer. 2017. PMID: 28143590 Free PMC article.
-
A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial.Eur J Clin Nutr. 2019 Sep;73(9):1224-1235. doi: 10.1038/s41430-018-0386-5. Epub 2019 Jan 15. Eur J Clin Nutr. 2019. PMID: 30647436 Clinical Trial.
References
-
- Lean ME. Pathophysiology of obesity. Proc Nutr Soc. Department of Human Nutrition, University of Glasgow, Glasgow Royal Infirmary, UK. mej.lean@clinmed.gla.ac.uk; 2000;59: 331–336. - PubMed
-
- Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121: 91–100. Available: http://www.ncbi.nlm.nih.gov/pubmed/11438497. - PubMed
-
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic Fatty Liver, Steatohepatitis, and the Metabolic Syndrome. Hepatology. 2003;37: 917–923. - PubMed
-
- Angulo P. Obesity and Nonalcoholic Fatty Liver Disease. Nutr Rev. 2007;65: 57–63. - PubMed
-
- Farrell GC, Larter CZ. Nonalcoholic Fatty Liver Disease: From Steatosis to Cirrhosis. Hepatology. 2006;43: S99–S112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

