Biphasic CD8+ T-Cell Defense in Simian Immunodeficiency Virus Control by Acute-Phase Passive Neutralizing Antibody Immunization
- PMID: 27122584
- PMCID: PMC4936138
- DOI: 10.1128/JVI.00557-16
Biphasic CD8+ T-Cell Defense in Simian Immunodeficiency Virus Control by Acute-Phase Passive Neutralizing Antibody Immunization
Abstract
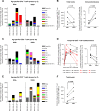
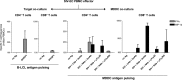
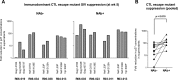
Identifying human immunodeficiency virus type 1 (HIV-1) control mechanisms by neutralizing antibodies (NAbs) is critical for anti-HIV-1 strategies. Recent in vivo studies on animals infected with simian immunodeficiency virus (SIV) and related viruses have shown the efficacy of postinfection NAb passive immunization for viremia reduction, and one suggested mechanism is its occurrence through modulation of cellular immune responses. Here, we describe SIV control in macaques showing biphasic CD8(+) cytotoxic T lymphocyte (CTL) responses following acute-phase NAb passive immunization. Analysis of four SIVmac239-infected rhesus macaque pairs matched with major histocompatibility complex class I haplotypes found that counterparts receiving day 7 anti-SIV polyclonal NAb infusion all suppressed viremia for up to 2 years without accumulating viral CTL escape mutations. In the first phase of primary viremia control attainment, CD8(+) cells had high capacities to suppress SIVs carrying CTL escape mutations. Conversely, in the second, sustained phase of SIV control, CTL responses converged on a pattern of immunodominant CTL preservation. During this sustained phase of viral control, SIV epitope-specific CTLs showed retention of phosphorylated extracellular signal-related kinase (ERK)(hi)/phosphorylated AMP-activated protein kinase (AMPK)(lo) subpopulations, implying their correlation with SIV control. The results suggest that virus-specific CTLs functionally boosted by acute-phase NAbs may drive robust AIDS virus control.
Importance: In early HIV infection, NAb responses are lacking and CTL responses are insufficient, which leads to viral persistence. Hence, it is important to identify immune responses that can successfully control such HIV replication. Here, we show that monkeys receiving NAb passive immunization in early SIV infection strictly control viral replication for years. Passive infusion of NAbs with CTL cross-priming capacity resulted in induction of functionally boosted early CTL responses showing enhanced suppression of CTL escape mutant virus replication. Accordingly, the NAb-infused animals did not show accumulation of viral CTL escape mutations during sustained SIV control, and immunodominant CTL responses were preserved. This early functional augmentation of CTLs by NAbs provides key insights into the design of lasting and viral escape mutation-free protective immunity against HIV-1 infection.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Post-infection immunodeficiency virus control by neutralizing antibodies.PLoS One. 2007 Jun 20;2(6):e540. doi: 10.1371/journal.pone.0000540. PLoS One. 2007. PMID: 17579714 Free PMC article.
-
Limited impact of passive non-neutralizing antibody immunization in acute SIV infection on viremia control in rhesus macaques.PLoS One. 2013 Sep 9;8(9):e73453. doi: 10.1371/journal.pone.0073453. eCollection 2013. PLoS One. 2013. PMID: 24039947 Free PMC article.
-
[In vivo protective mechanisms of neutralizing antibodies against simian immunodeficiency virus replicatio].Uirusu. 2021;71(1):87-96. doi: 10.2222/jsv.71.87. Uirusu. 2021. PMID: 35526999 Japanese.
-
Anti-HIV adaptive immunity: determinants for viral persistence.Rev Med Virol. 2008 Sep-Oct;18(5):293-303. doi: 10.1002/rmv.577. Rev Med Virol. 2008. PMID: 18416450 Review.
-
Cytotoxic T lymphocytes specific for the simian immunodeficiency virus.Immunol Rev. 1999 Aug;170:127-34. doi: 10.1111/j.1600-065x.1999.tb01334.x. Immunol Rev. 1999. PMID: 10566147 Review.
Cited by
-
Patterns of HIV/SIV Prevention and Control by Passive Antibody Immunization.Front Microbiol. 2016 Nov 2;7:1739. doi: 10.3389/fmicb.2016.01739. eCollection 2016. Front Microbiol. 2016. PMID: 27853456 Free PMC article. Review.
-
AMP-Activated Protein Kinase and Host Defense against Infection.Int J Mol Sci. 2018 Nov 6;19(11):3495. doi: 10.3390/ijms19113495. Int J Mol Sci. 2018. PMID: 30404221 Free PMC article. Review.
-
Antiviral Functions of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific IgG Antibodies: Effects of Antiretroviral Therapy and Implications for Therapeutic HIV-1 Vaccine Design.Front Immunol. 2017 Jul 4;8:780. doi: 10.3389/fimmu.2017.00780. eCollection 2017. Front Immunol. 2017. PMID: 28725225 Free PMC article. Review.
-
Association of lymph-node antigens with lower Gag-specific central-memory and higher Env-specific effector-memory CD8(+) T-cell frequencies in a macaque AIDS model.Sci Rep. 2016 Jul 25;6:30153. doi: 10.1038/srep30153. Sci Rep. 2016. PMID: 27452272 Free PMC article.
-
A Novel Immunogen Selectively Eliciting CD8+ T Cells but Not CD4+ T Cells Targeting Immunodeficiency Virus Antigens.J Virol. 2020 Mar 31;94(8):e01876-19. doi: 10.1128/JVI.01876-19. Print 2020 Mar 31. J Virol. 2020. PMID: 32024773 Free PMC article.
References
-
- Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi:10.1128/JVI.01708-08. - DOI - PMC - PubMed
-
- Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. 2010. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med 16:1117–1119. doi:10.1038/nm.2233. - DOI - PMC - PubMed
-
- Jaworski JP, Kobie J, Brower Z, Malherbe DC, Landucci G, Sutton WF, Guo B, Reed JS, Leon EJ, Engelmann F, Zheng B, Legasse A, Park B, Dickerson M, Lewis AD, Colgin LM, Axthelm M, Messaoudi I, Sacha JB, Burton DR, Forthal DN, Hessell AJ, Haigwood NL. 2013. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J Virol 87:10447–10459. doi:10.1128/JVI.00049-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

